What Makes Cells Move?
Steven J Wolf | March 2014
Cell migration or movement is fundamental to life itself. In fact, such movement is essential from the earliest stages of embryo development through to processes such as wound healing. Unfortunately, when this orchestrated movement goes awry, as occurs in cancer cells, deadly tumors may develop. As such, understanding cell movement is very relevant to modern medicine and scientists are focusing their efforts on fully understanding this fundamental process.
Hirata H, Tatsumi H, Lim CT and Sokabe M. Force-dependent vinculin binding to talin in live cells: a crucial step in anchoring the actin cytoskeleton to focal adhesions. Am J Physiol Cell Physiol, 2014, 306(6):C607-20
Identifying key components of the cellular engine in living cells
In work recently published by Dr Hiroaki Hirata, a postdoctoral fellow in the laboratory of Prof Chwee Teck Lim of the Mechanobiology Institute, National University of Singapore, and Prof Masahiro Sokabe of the Nagoya University Graduate School of Medicine in Japan, a key element in the machinery that drives cell movement was described for the first time in living cells.
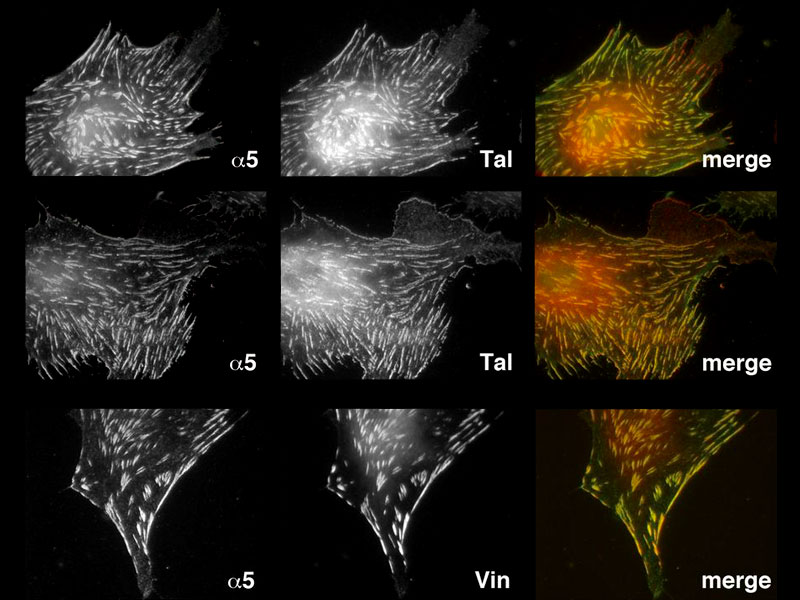
It is well established that individual cells possess distinct structures, known as focal adhesions, which allow them to grip onto a surface and generate force internally. Inside the cell, focal adhesions are physically linked to a network of filaments or cables, composed of a protein called actin, that form near the cell membrane. These are highly dynamic and are constantly undergoing assembly and disassembly. Due to their dynamic nature, as well as the activity of a protein known as myosin, the actin filaments are constantly moving inwards, towards the center of the cell.
Force-dependent binding of vinculin to talin has been reported in in vitro and in silico studies. We have, for the first time demonstrated that this force- dependent binding indeed occurs at focal adhesions in living cells. –Hiroaki Hirata, MBI research fellow
The question that scientists have long sought to understand is how a moving actin filament network can be physically linked to a fixed adhesion site, and how this linkage generates cell movement. Previous work had shown that a protein known as talin could theoretically provide this link, however this protein could only sustain a small amount of internal force, and would ‘slip’ with any force larger than 2pN. It was akin to playing tug-of-war with a lubricated rope. However, the actin binding protein talin was also known to possess multiple vinculin binding sites and the force-dependent binding of vinculin to talin had been observed in both in vitro and in silico studies. It had just never been observed in living cells.
Hirata et al have now shown that indeed, vinculin binding to talin does occur in living cells. Although talin will slip beyond 2pN, this encourages the accumulation of vinculin at the focal adhesion site, and, before the link slips, multiple vinculin proteins bind to the complex, forming an integrin- talin-vinculin-actin link. Each vinculin molecule was shown to withstand 2.5pN of force. With multiple vinculin proteins able to bind to this complex, a far greater force was sustained, and this is sufficient to tether the actin filament network to focal adhesions.
Although the actin filaments no longer move inwards, they are still growing near the cell membrane. In the cell, the membrane protrudes outwards around the growing filaments. Eventually new adhesions form at the tip of the protrusion, allowing the cell to grab onto the substrate surface. The protrusion thus becomes the front of the cell, and the rear must follow, allowing the cell to move forwards. Traction has been harnessed and the cell will inch its way forward, testing and responding to the stiffness of its substrate, until it reaches its destination.
With cell motility particularly relevant to cancer treatment, burns treatment and organ development, our understanding of cell motility and the machinery that mediate this process is particularly important. With further understanding of these processes, the researchers at MBI hope to identify new technologies and therapies targeted at encouraging, or preventing, cell motility.