What steps are involved in the myosin powerstroke?
Each myosin motor protein possesses ATPase activity and functions in a cyclical manner that couples ATP binding and hydrolysis to a conformational change in the protein. This process is known as the ‘powerstroke cycle’ (reviewed in [1][2][3]) and is outlined in the steps below using myosin II as an example.
The direction in which the actin filament will be moved is dictated by the structural orientation of myosin in relation to the filament. A complete round of ATP hydrolysis produces a single ‘step’ or movement of myosin along the actin filament. This process is regulated by changes in the concentration of intracellular free calcium (reviewed in [4]). The steps involved are detailed below:
- At the end of the previous round of movement and the start of the next cycle, the myosin head lacks a bound ATP and it is attached to the actin filament in a very short-lived conformation known as the ‘rigor conformation’.
- ATP binding to the myosin head domain induces a small conformational shift in the actin-binding site that reduces its affinity for actin and causes the myosin head to release the actin filament.
- ATP binding also causes a large conformational shift in the ‘lever arm’ of myosin that bends the myosin head into a position further along the filament. ATP is then hydrolysed, leaving the inorganic phosphate and ADP bound to myosin.
- The myosin head makes weak contact with the actin filament and a slight conformational change occurs on myosin that promotes the release of the inorganic phosphate.
- The release of inorganic phosphate reinforces the binding interaction between myosin and actin and subsequently triggers the ‘power stroke’. The power stroke is the key force-generating step used by myosin motor proteins. Forces are generated on the actin filament as the myosin protein reverts back to its original conformation.
- As myosin regains its original conformation, the ADP is released, but the myosin head remains tightly bound to the filament at a new position from where it started, thereby bringing the cycle back to the beginning.
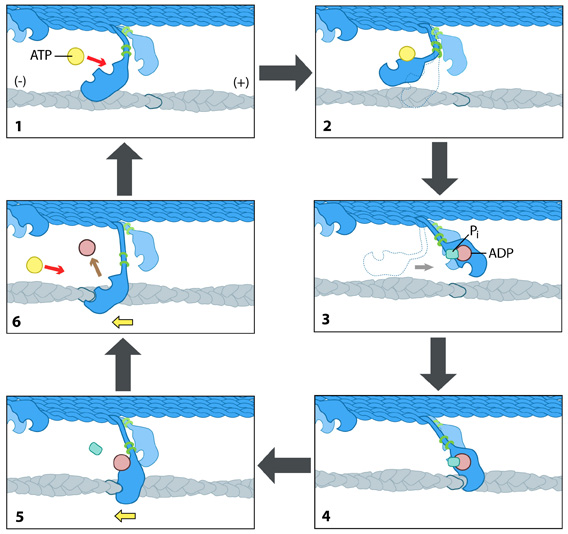
The “power stroke” mechanism for myosin movement along actin filaments.
References
- Vale RD, and Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science 2000; 288(5463):88-95. [PMID: 10753125]
- Volkmann N, and Hanein D. Actomyosin: law and order in motility. Curr. Opin. Cell Biol. 2000; 12(1):26-34. [PMID: 10679363]
- Hwang W, and Lang MJ. Mechanical design of translocating motor proteins. Cell Biochem. Biophys. 2009; 54(1-3):11-22. [PMID: 19452133]
- Ebashi S, and Endo M. Calcium ion and muscle contraction. Prog. Biophys. Mol. Biol. 1968; 18:123-83. [PMID: 4894870]


