What steps are involved in the formation of focal adhesions?
The assembly of focal adhesions is highly regulated, with protein recruitment occurring in a sequential manner [1], and resulting in structures that are organized in specific layers that corresponding to the following functions:
- Receptor-matrix binding
- Linkage to actin cytoskeleton and force transduction
- Intracellular signal transduction
- Actin polymerization and regulation
Focal adhesions act as molecular clutches that provide grip to the substrate for the lamellipodium to protrude forward during motility. Their formation is highly dependent on the lamellipodial actin flow [2].
Focal adhesion formation is initiated by receptor-matrix binding along the cell periphery at the leading edge. These early complexes, hitherto referred as “nascent adhesions”, initially attach to actin filaments via adaptor proteins such as talin [3][4]. At the lamellipodium-lamellum interface, unstable adhesions disappear and stable ones start to elongate in a centripetal fashion along the direction of actin retrograde flow. Growing adhesions increase the traction on the substrate, slow down the actin retrograde flow [2][5], aid force transduction along the attached actin bundles and in turn, reinforce the linkage to actin by recruitment of additional scaffolding and signaling components [6][7] (reviewed in [8]).
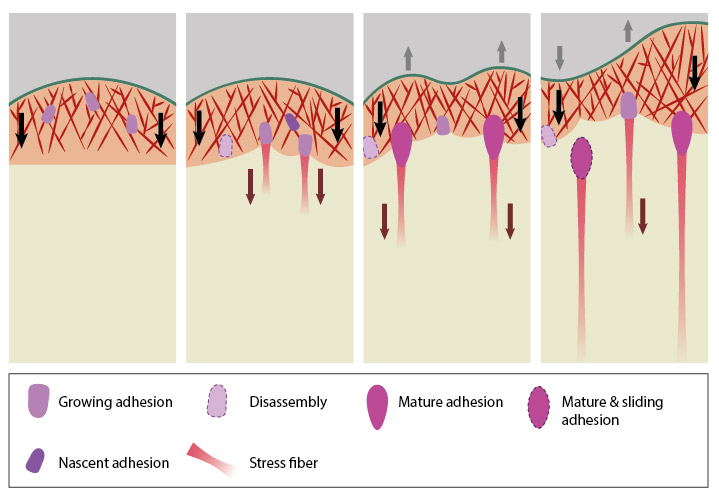
The four panels represent roughly the different steps in the formation and disassembly of focal adhesions. The lamellipodium is distinguished from the lamellum by a color difference; the actin filaments represent the retrograde flow and the black arrows indicate its characteristic centripetal flow. From left: Panel 1 shows formation of nascent adhesions at the cell periphery. In Panel 2, some adhesions attach to stress fibers and grow in size while some disassemble (dotted outline) at the lamellipodium-lamellum boundary. Maturation happens upon increase in tension along the stress fibers (Panel 3). The protrusions of the cell edge are shown as grey arrows corresponding to points of adhesion maturation (Panels 3 and 4). Panel 4 shows sliding of adhesion and hence slight retraction of the respective section of the cell edge compared to Panel 3. Decrease in retrograde flow at points of adhesions is marked by the dipping of the lamellipodial border. Adapted from [4, 8].
During the transition from nascent to mature adhesions, the morphology of the focal adhesion changes from a symmetric diffraction-limited spot to that of a polar, elongated formation with a distal tip (‘toe’) and a proximal end (‘heel’) (reviewed in [13]). Newly formed actin bundles grow from the heel of mature focal adhesions. The proteins that participate in and the series of events that lead to this dynamic reorganization are still unclear.
It should to be noted that, reinforcement of the adhesion structure happens alongside assembly as and when particular components get recruited and is not restricted to any particular stage. Similarly, signaling transduction also happens throughout the life-cycle of adhesions.
Since focal adhesion growth correlates with cell movement relative to the substrate, focal adhesions appear to move from the periphery to the cell center as they grow [14]. At each stage they undergo turnover after a certain time period (reviewed in [15]). Within a particular adhesion, the bond strengths between ligand-receptor-cytoskeleton are preferentially stable at the leading edge and weaken towards the rear, where disassembly happens [16]. Earlier adhesions exert high propulsive forces which aid cell migration. This decreases at later stages such that upon maturation, the adhesions effectively act as passive anchorage points [17]. Also, nascent adhesions are mostly non-motile while late adhesions can reorient themselves in response to forces experienced [16][18].
References
- Zaidel-Bar R, Cohen M, Addadi L, and Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem. Soc. Trans. 2004; 32(Pt3):416-20. [PMID: 15157150]
- Alexandrova AY, Arnold K, Schaub S, Vasiliev JM, Meister J, Bershadsky AD, and Verkhovsky AB. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE 2008; 3(9):e3234. [PMID: 18800171]
- Jiang G, Giannone G, Critchley DR, Fukumoto E, and Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 2003; 424(6946):334-7. [PMID: 12867986]
- Giannone G, Mège R, and Thoumine O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol. 2009; 19(9):475-86. [PMID: 19716305]
- Gardel ML, Sabass B, Ji L, Danuser G, Schwarz US, and Waterman CM. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J. Cell Biol. 2008; 183(6):999-1005. [PMID: 19075110]
- Zamir E, and Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J. Cell. Sci. 2001; 114(Pt 20):3583-90. [PMID: 11707510]
- Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Döbereiner H, Freund Y, Borisy G, and Sheetz MP. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell 2007; 128(3):561-75. [PMID: 17289574]
- Geiger B, Spatz JP, and Bershadsky AD. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009; 10(1):21-33. [PMID: 19197329]
- Giannone G, and Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006; 16(4):213-23. [PMID: 16529933]
- Le Clainche C, and Carlier M. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 2008; 88(2):489-513. [PMID: 18391171]
- Laukaitis CM, Webb DJ, Donais K, and Horwitz AF. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J. Cell Biol. 2001; 153(7):1427-40. [PMID: 11425873]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, and Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004; 6(2):154-61. [PMID: 14743221]
- Wolfenson H, Henis YI, Geiger B, and Bershadsky AD. The heel and toe of the cell’s foot: a multifaceted approach for understanding the structure and dynamics of focal adhesions. Cell Motil. Cytoskeleton 2009; 66(11):1017-29. [PMID: 19598236]
- Smilenov LB, Mikhailov A, Pelham RJ, Marcantonio EE, and Gundersen GG. Focal adhesion motility revealed in stationary fibroblasts. Science 1999; 286(5442):1172-4. [PMID: 10550057]
- Gardel ML, Schneider IC, Aratyn-Schaus Y, and Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu. Rev. Cell Dev. Biol. 2010; 26:315-33. [PMID: 19575647]
- Nishizaka T, Shi Q, and Sheetz MP. Position-dependent linkages of fibronectin- integrin-cytoskeleton. Proc. Natl. Acad. Sci. U.S.A. 2000; 97(2):692-7. [PMID: 10639141]
- Beningo KA, Dembo M, Kaverina I, Small JV, and Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 2001; 153(4):881-8. [PMID: 11352946]
- Partridge MA, and Marcantonio EE. Initiation of attachment and generation of mature focal adhesions by integrin-containing filopodia in cell spreading. Mol. Biol. Cell 2006; 17(10):4237-48. [PMID: 16855018]


