What steps are involved in Lamellipodia assembly?
The lamellipodia is a distinct region of the cell that facilitates cell motility and various mechanosensing mechanisms. Lamellipodium assembly can be described in a series of defined steps, many of which involve processes related to cytoskeletal dynamics, and which utilize several “functional modules”.
The actin cytoskeleton plays an essential role in the formation and function of the lamellipodia. Lamellipodial actin filaments are highly dynamic, especially compared to those of the lamella [1] and it is due to their dynamic nature, and the constant cycles of actin filament polymerization and actin filament depolymerization that the protrusive force required to stretch the membrane and allow the lamellipodia to spread, is generated.
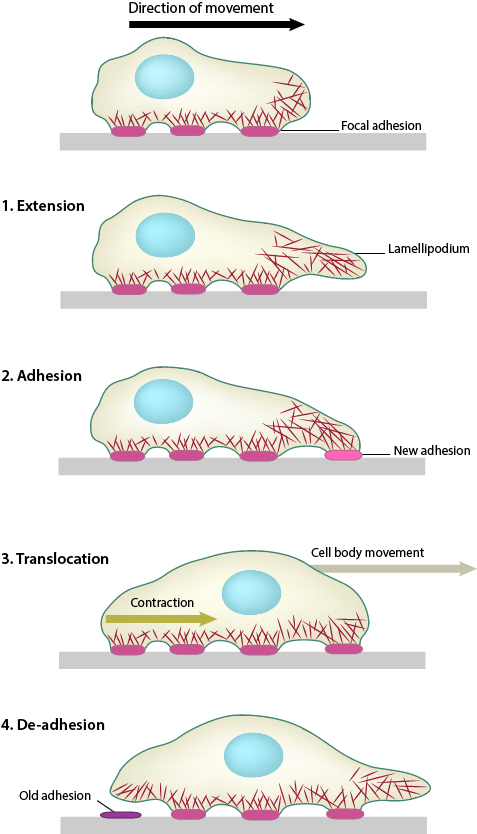
1. Polymerization of actin filaments at the leading edge is translated into protrusive force. 2. Membrane protrusion facilitates the binding of transmembrane cell surface receptors to the substratum components. New adhesions are rapidly linked to the network of actin filaments. 3. The combined activity of retrograde actin movement and contractile forces produced by stress fibers generate tension to pull the cell body forward. 4. The forces produced by the contractile network combined with actin filament and focal adhesion disassembly, helps to retract the trailing cell edge. (Note: the “space” between the cell and the substrate [shown as a gray bar] is exaggerated in this diagram).
The above mentioned activities produce net forward/backward cell movement or spreading of the plasma membrane in a specific direction (aka polarized movement).
What biopysical or biochemical factors drive lamellipodia function?
It should be noted however that the specific biophysical and biochemical parameters that are altered at each step have been difficult to measure due to the wide variety of motile structures that can be found in each cell at any given time. For example, migratory fibroblasts exhibit lamellipodia protrusion and retraction, ruffling, filopodial protrusion and retraction, bleb protrusion and retraction, trailing edge retraction, and quiescence in neighboring regions of the cell edge [2].
Fortunately, recent advances in technological systems that measure the temporal and spatial movement of single proteins in specific cell structures, whole cells, and tissues has greatly expanded our mechanistic understanding of cell motility and the composite functional modules [2][3][4][5][6] (reviewed in [7]). Surprisingly, recent work has suggested that the basic mechanism for polarization and directional movement lies in the microtubules, which can be modified by their interaction with the actin-myosin system and cell-substrate adhesions [8]. Although there are numerous details that remain unresolved, it is abundantly clear that mechanical mechanisms are essential for coordinating the physical and biochemical processes that determine cell shape and locomotion.
References
- Ridley AJ. Life at the leading edge. Cell 2011; 145(7):1012-22. [PMID: 21703446]
- Dubin-Thaler BJ, Hofman JM, Cai Y, Xenias H, Spielman I, Shneidman AV, David LA, Döbereiner H, Wiggins CH, and Sheetz MP. Quantification of cell edge velocities and traction forces reveals distinct motility modules during cell spreading. PLoS ONE 2008; 3(11):e3735. [PMID: 19011687]
- Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Döbereiner H, Freund Y, Borisy G, and Sheetz MP. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell 2007; 128(3):561-75. [PMID: 17289574]
- Döbereiner H, Dubin-Thaler BJ, Hofman JM, Xenias HS, Sims TN, Giannone G, Dustin ML, Wiggins CH, and Sheetz MP. Lateral membrane waves constitute a universal dynamic pattern of motile cells. Phys. Rev. Lett. 2006; 97(3):038102. [PMID: 16907546]
- Alexandrova AY, Arnold K, Schaub S, Vasiliev JM, Meister J, Bershadsky AD, and Verkhovsky AB. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE 2008; 3(9):e3234. [PMID: 18800171]
- Tsukada Y, Aoki K, Nakamura T, Sakumura Y, Matsuda M, and Ishii S. Quantification of local morphodynamics and local GTPase activity by edge evolution tracking. PLoS Comput. Biol. 2008; 4(11):e1000223. [PMID: 19008941]
- Vogel V, and Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006; 7(4):265-75. [PMID: 16607289]
- Shutova MS, Alexandrova AY, and Vasiliev JM. Regulation of polarity in cells devoid of actin bundle system after treatment with inhibitors of myosin II activity. Cell Motil. Cytoskeleton 2008; 65(9):734-46. [PMID: 18615701]


