What is Zyxin?
Zyxin is enriched along actin filaments, stress fiber bundles, and at cell-cell or cell-matrix adhesion sites [1][2].
Zyxin is specifically found in more mature adhesions [3] and its absence in early adhesions is commonly used to distinguish the ‘age’ of an adhesion [4]. The main function of zyxin is to form a bridge between the adhesion components at the cell membrane and the internal cytoskeleton (reviewed in [5]). Zyxin is vital for coordinating matrix-dependent cues with actin dynamics; for example, within stress fibers and focal adhesions (FAs), zyxin acts as a mechanosensor that binds to areas where forces are applied [6][7][8]. Not only is zyxin binding proportional to the mechanical force (e.g. decreased traction reduces zyxin-binding) but its stability at adhesion sites is tension-dependent [9][10].
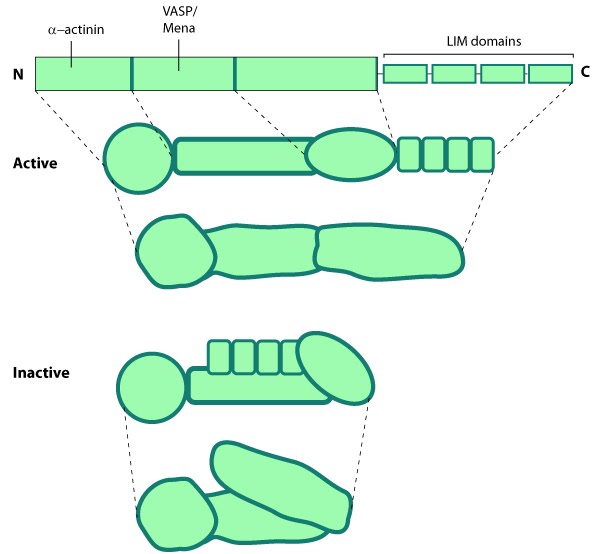
This schematic diagram illustrates the molecular organization of zyxin and provides examples for how zyxin is represented in figures throughout this resource.
Cellular adaptation to mechanical stress also involves redistribution of zyxin from FAs to stress fibers, which causes stress fiber thickening [11]. Mislocalization of zyxin leads to defects in cell migration and spreading [12][13] and its absence leads to increased cellular motility [14] presumably through reduced adhesive strength [15]. Zyxin influences actin organization and assembly around FAs by recruiting Ena/VASP [13][14][16]; Ena/VASP may subsequently generate new actin filaments by an unknown mechanism that is initiation factor-independent, however, this has not been demonstrated in intact cells [17].
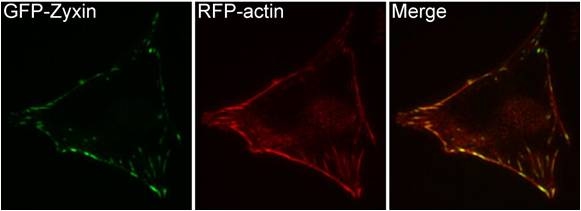
A NIH3T3 cell, plated on a collagen coated glass slide and transfected with RFP-actin and GFP-zyxin. The basal surface of the cell was imaged using TIRF microscopy, on an Olympus IX81 Inverted microscope at 60x magnification. [Image captured by Machiyama Hiroaki, Mechanobiology Institute, Singapore]
- Crawford AW, and Beckerle MC. Purification and characterization of zyxin, an 82,000-dalton component of adherens junctions. J. Biol. Chem. 1991; 266(9):5847-53. [PMID: 2005121]
- Sadler I, Crawford AW, Michelsen JW, and Beckerle MC. Zyxin and cCRP: two interactive LIM domain proteins associated with the cytoskeleton. J. Cell Biol. 1992; 119(6):1573-87. [PMID: 1469049]
- Beningo KA, Dembo M, Kaverina I, Small JV, and Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 2001; 153(4):881-8. [PMID: 11352946]
- Zaidel-Bar R, Ballestrem C, Kam Z, and Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell. Sci. 2003; 116(Pt 22):4605-13. [PMID: 14576354]
- Beckerle MC. Zyxin: zinc fingers at sites of cell adhesion. Bioessays 1997; 19(11):949-57. [PMID: 9394617]
- Vogel V, and Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006; 7(4):265-75. [PMID: 16607289]
- Curtis A, and Riehle M. Tissue engineering: the biophysical background. Phys Med Biol 2001; 46(4):R47-65. [PMID: 11324976]
- Spatz JP, and Geiger B. Molecular engineering of cellular environments: cell adhesion to nano-digital surfaces. Methods Cell Biol. 2007; 83:89-111. [PMID: 17613306]
- Lele TP, Pendse J, Kumar S, Salanga M, Karavitis J, and Ingber DE. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J. Cell. Physiol. 2006; 207(1):187-94. [PMID: 16288479]
- Colombelli J, Besser A, Kress H, Reynaud EG, Girard P, Caussinus E, Haselmann U, Small JV, Schwarz US, and Stelzer EHK. Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J. Cell. Sci. 2009; 122(Pt 10):1665-79. [PMID: 19401336]
- Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, and Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 2005; 171(2):209-15. [PMID: 16247023]
- Drees BE, Andrews KM, and Beckerle MC. Molecular dissection of zyxin function reveals its involvement in cell motility. J. Cell Biol. 1999; 147(7):1549-60. [PMID: 10613911]
- Drees B, Friederich E, Fradelizi J, Louvard D, Beckerle MC, and Golsteyn RM. Characterization of the interaction between zyxin and members of the Ena/vasodilator-stimulated phosphoprotein family of proteins. J. Biol. Chem. 2000; 275(29):22503-11. [PMID: 10801818]
- Hoffman LM, Jensen CC, Kloeker S, Wang CA, Yoshigi M, and Beckerle MC. Genetic ablation of zyxin causes Mena/VASP mislocalization, increased motility, and deficits in actin remodeling. J. Cell Biol. 2006; 172(5):771-82. [PMID: 16505170]
- Ngu H, Feng Y, Lu L, Oswald SJ, Longmore GD, and Yin FC. Effect of focal adhesion proteins on endothelial cell adhesion, motility and orientation response to cyclic strain. Ann Biomed Eng 2009; 38(1):208-22. [PMID: 19856213]
- Nix DA, Fradelizi J, Bockholt S, Menichi B, Louvard D, Friederich E, and Beckerle MC. Targeting of zyxin to sites of actin membrane interaction and to the nucleus. J. Biol. Chem. 2001; 276(37):34759-67. [PMID: 11395501]
- Fradelizi J, Noireaux V, Plastino J, Menichi B, Louvard D, Sykes C, Golsteyn RM, and Friederich E. ActA and human zyxin harbour Arp2/3-independent actin-polymerization activity. Nat. Cell Biol. 2001; 3(8):699-707. [PMID: 11483954]


