What is the role of Rho GTPases in the regulation of focal adhesion assembly?
Synergistic integrin-syndecan signaling
The rate and extent of focal adhesion formation and maturation are regulated by factors such as synergistic integrin–syndecan signaling and alternating activation cycles of Rho (Rac1, Cdc42 and RhoA) GTPases. Neither syndecan nor integrin is capable of independently supporting cell adhesion or spreading. Despite the cooperativity of integrin-syndecan pairs in various contexts (reviewed in [1]), recent studies have established synergistic signaling by integrin β1 and syndecan-4; they play cooperative yet distinct roles in cell spreading and maturation of adhesions as well as directional migration respectively [2][3]. The receptors co-localize in early adhesion sites at the leading edge with ligand binding by both receptors (e.g fibronectin binds via cell binding domain [RGD to integrin and via HepII domain to syndecan) being necessary for downstream signaling [4][5]. This is crucial as the cell polarity and migration is determined by differentially regulating signals at the leading and trailing edges.
Migration comprises of cycles of membrane protrusion, attachment, and cytoskeletal contraction, which causes forward movement. Immobilization of the integrin ligand is absolutely necessary to generate tension for adhesion formation and actin bundling while syndecan signaling primarily helps sense the environment for membrane protrusion.
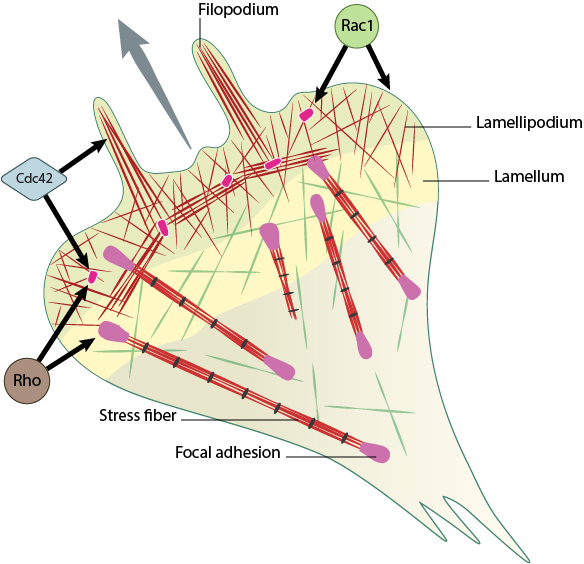
Rho family GTPases Cdc42, Rac1 and Rho act at different regions in a cell (indicated by arrows) to orchestrate migration. Cdc42 generally controls the cell polarity and the formation of filopodia and nascent focal adhesions (shown as yellow dots). Rho influences cell adhesion assembly and maturation, in addition to controlling stress fiber formation and contractile activity. Rac1 primarily controls actin assembly and nascent adhesion formation in the lamellipodium.
RhoGTPase dynamics during focal adhesion assembly
Localized signaling happens through alternating activation cycles of GTPases Rac1 (lamellipodium) and/or Cdc42 (filopodium) and RhoA, regulated by protein kinase pathways at the leading edge [6] (reviewed in [7][8]). Stable adhesion induced by Rac1 may initially support tension, which allows RhoA-mediated contractility and pulling forces to impart stability on the bonds, thereby generating subsequent signals that are disseminated to the rest of the cell or axon [9][10][11][12][13]; these signals serve as feedback loops to restrict the direction of protrusion and reduce local activity of Rac1 [14], [15]. It is to be noted that either receptor contributes to the regulation of both GTPases, however, Rac1 is primarily influenced by syndecan-4 [16]. Coordination of such complex signaling is rendered by guidance signals.
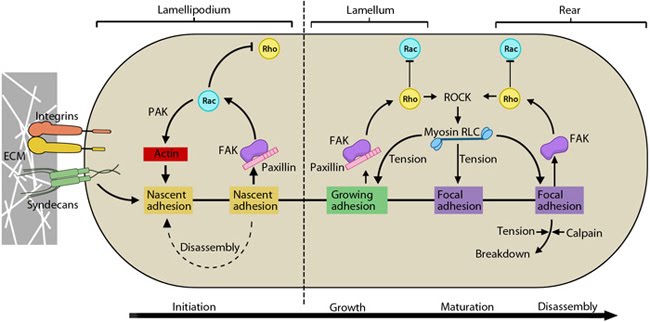
This schematic highlights the signaling pathways that play a significant role in mediating GTPase-regulated protrusion in the lamellipodia and the differential adhesion dynamics various regions of the cell. FAK signaling is important at all stages of the adhesion life cycle while kinases such as PAK and ROCK influence later stages by promoting actomyosin contractility. PAK is highlighted in green during maturation to indicate that is it activated earlier by Rac and not by Rho. Adapted from [17, 18].
While α5β1 integrin activates Rac1 by regulating both localization to leading edge and GTP-loading, syndecan-4 influences only the GTP-loading (reviewed in [1]). Upon ligand binding, integrin signaling recruits GEFs for Rac1 and/or Cdc42 in lamellipodia and filopodia respectively [17][18][19][20]. These initiate basal level Rac1 activity required for initial cell spreading [21], coordinated by Src–FAK signaling pathway [22] (reviewed in [23][24]). Further, liganded integrins retain active Rac1 at the leading edge by mediating a transient lipid redistribution which localizes GTP-Rac1 to the membrane [25].
During this relocation, the interaction of Rac1 with Rho-GDI is disrupted, allowing p21-activated kinase (PAK) coupling [26][27]. In the lamellipodium, PAK promotes actin polymerization by inactivating cofilin and aids spreading by suppressing local myosin activity periodically [18][28][29]. It also aids actin reorganization in the lamellae [29].
Upon engagement, syndecan-4 forms a ternary complex with PKCα and PIP2 [30], [31] and its oligomerization leads to activation of PKCα [32]. This is a critical step for further GTP-loading of Rac1, restricting Rac1 activity to the leading edge and environment sensing for directional migration [16]. These facilitate downstream signaling for Rac1-mediated actin protrusion [33].
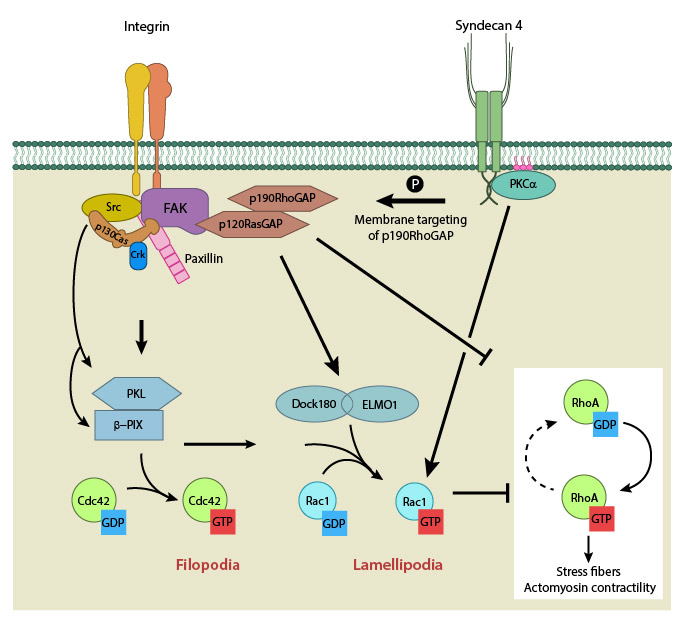
Integrin signaling and nascent adhesion formation recruits GEFs such as Dock 180 and PIX for Rac and Cdc42 at the leading edge, activating them. It also inactivates p190RhoGAP while PKC α activation downstream of syndecan-4 sequesters it the membrane to suppress Rho activation. PIP2 is indicated on the membrane in pink. PKCα activation also aids further Rac1 activation and ensures sustenance of Rac1 activity at the leading edge by lipid distributions (not shown). Adapted from [18].
It is highly critical to suppress contractile signals during protrusion to aid forward movement and adhesion turnover. This is achieved again through convergent receptor signaling that retains RhoA inactive during Rac1 activity at the leading edge [34][35]. On integrin β1 engagement, FAK phosphorylates and inactivates p190RhoGAP required for RhoA activation (essential role) [36][37]. It then gets docked in the membrane fraction by binding p120RasGAP and FAK [38], and syndecan-4-mediated redistribution to membrane ruffles [39][40] (modulatory role). Further, p190RhoGAP phosphorylation triggers another wave of integrin-dependent lipid distribution that sustains RhoA suppression until the cell is fully spread.
Rho activation during late spreading
The precise reciprocal feedback mechanism through which RhoA is activated in the lamellae during adhesion maturation/disassembly remained unclear until recently. However several lines of evidence exist for involvement of integrin– and syndecan-signaling pathways.
Besides reorganizing actin, Rac-activated PAK also phosphorylates the regulatory light chain (RLC) of myosin II, thus activating bundling of actin and the contractile mechanism. Myosin IIA/Myosin IIB-mediated actomysoin bundling generates stable adhesions, inhibit Rac-GEFs in the vicinity by modifying adhesion components that aid their recruitment and thus establish the cell rear [41]. In a force-dependent manner, Rho-specific GEFs have been shown to get activated and recruited to focal adhesions through FAK and Fyn [42][43] (reviewed in [37]). Thus integrin-signaling pathway activates RhoA. Similarly, a syndecan-4 dependent pathway has been shown for the formation and maintenance of stress fibres, and focal adhesion maturation. Upon syndecan-4 clustering, GTP-loading of RhoA increases in a PKCα-dependent manner [44].
Activation of RhoA further enhances contractility and builds cellular tension through the Rho kinase, ROCK which sustains the myosin RLC phosphorylation [45][46](reviewed in [47]). Tension-dependent decrease of Rac activity has been demonstrated [48][ 16129884[/cite] and is believed to happen through stimulation of a Rac-GAP, ARHGAP22 by the Rho kinase, ROCK [49]. Similarly CdGAP, has been shown to inhibit lamellipodial protrusion and is suggested to do so via its actions on both Rac and Cdc42 [17][50].
References
- Morgan MR, Humphries MJ, and Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 2007; 8(12):957-69. [PMID: 17971838]
- Thodeti CK, Albrechtsen R, Grauslund M, Asmar M, Larsson C, Takada Y, Mercurio AM, Couchman JR, and Wewer UM. ADAM12/syndecan-4 signaling promotes beta 1 integrin-dependent cell spreading through protein kinase Calpha and RhoA. J. Biol. Chem. 2002; 278(11):9576-84. [PMID: 12509413]
- Bass MD, Morgan MR, and Humphries MJ. Integrins and syndecan-4 make distinct, but critical, contributions to adhesion contact formation. Soft Matter 2007; 3(3):372-376. [PMID: 19458789]
- Woods A, Longley RL, Tumova S, and Couchman JR. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch. Biochem. Biophys. 2000; 374(1):66-72. [PMID: 10640397]
- Clark RAF, An J, Greiling D, Khan A, and Schwarzbauer JE. Fibroblast migration on fibronectin requires three distinct functional domains. J. Invest. Dermatol. 2003; 121(4):695-705. [PMID: 14632184]
- Guo F, Debidda M, Yang L, Williams DA, and Zheng Y. Genetic deletion of Rac1 GTPase reveals its critical role in actin stress fiber formation and focal adhesion complex assembly. J. Biol. Chem. 2006; 281(27):18652-9. [PMID: 16698790]
- Burridge K, and Wennerberg K. Rho and Rac take center stage. Cell 2004; 116(2):167-79. [PMID: 14744429]
- Guilluy C, Garcia-Mata R, and Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011; 21(12):718-26. [PMID: 21924908]
- Lamoureux P, Buxbaum RE, and Heidemann SR. Direct evidence that growth cones pull. Nature 1989; 340(6229):159-62. [PMID: 2739738]
- Smith A, Sengupta K, Goennenwein S, Seifert U, and Sackmann E. Force-induced growth of adhesion domains is controlled by receptor mobility. Proc. Natl. Acad. Sci. U.S.A. 2008; 105(19):6906-11. [PMID: 18463289]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, and Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001; 3(5):466-72. [PMID: 11331874]
- Gomez TM, Robles E, Poo M, and Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science 2001; 291(5510):1983-7. [PMID: 11239161]
- Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, Chien S, Hahn KM, and Schwartz MA. Effects of cell tension on the small GTPase Rac. J. Cell Biol. 2002; 158(1):153-64. [PMID: 12105187]
- Woo S, and Gomez TM. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J. Neurosci. 2006; 26(5):1418-28. [PMID: 16452665]
- Rottner K, Hall A, and Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 1999; 9(12):640-8. [PMID: 10375527]
- Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, Mayer U, Ballestrem C, Spatz JP, and Humphries MJ. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J. Cell Biol. 2007; 177(3):527-38. [PMID: 17485492]
- Kurokawa K, Itoh RE, Yoshizaki H, Nakamura YOT, and Matsuda M. Coactivation of Rac1 and Cdc42 at lamellipodia and membrane ruffles induced by epidermal growth factor. Mol. Biol. Cell 2003; 15(3):1003-10. [PMID: 14699061]
- Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, and Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J. Cell Biol. 2006; 173(4):587-9. [PMID: 16717130]
- Kawasaki Y, Senda T, Ishidate T, Koyama R, Morishita T, Iwayama Y, Higuchi O, and Akiyama T. Asef, a link between the tumor suppressor APC and G-protein signaling. Science 2000; 289(5482):1194-7. [PMID: 10947987]
- Katoh H, and Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature 2003; 424(6947):461-4. [PMID: 12879077]
- Berrier AL, Martinez R, Bokoch GM, and LaFlamme SE. The integrin beta tail is required and sufficient to regulate adhesion signaling to Rac1. J. Cell. Sci. 2002; 115(Pt 22):4285-91. [PMID: 12376560]
- Sharma A, and Mayer BJ. Phosphorylation of p130Cas initiates Rac activation and membrane ruffling. BMC Cell Biol. 2008; 9:50. [PMID: 18793427]
- Huveneers S, and Danen EHJ. Adhesion signaling – crosstalk between integrins, Src and Rho. J. Cell. Sci. 2009; 122(Pt 8):1059-69. [PMID: 19339545]
- Parsons JT, Horwitz AR, and Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010; 11(9):633-43. [PMID: 20729930]
- del Pozo MA, Alderson NB, Kiosses WB, Chiang H, Anderson RGW, and Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science 2004; 303(5659):839-42. [PMID: 14764880]
- Del Pozo MA, Kiosses WB, Alderson NB, Meller N, Hahn KM, and Schwartz MA. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 2002; 4(3):232-9. [PMID: 11862216]
- Lu W, and Mayer BJ. Mechanism of activation of Pak1 kinase by membrane localization. Oncogene 1999; 18(3):797-806. [PMID: 9989831]
- Giannone G, Dubin-Thaler BJ, Döbereiner H, Kieffer N, Bresnick AR, and Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell 2004; 116(3):431-43. [PMID: 15016377]
- Delorme V, Machacek M, DerMardirossian C, Anderson KL, Wittmann T, Hanein D, Waterman-Storer C, Danuser G, and Bokoch GM. Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev. Cell 2007; 13(5):646-62. [PMID: 17981134]
- Oh ES, Woods A, and Couchman JR. Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C. J. Biol. Chem. 1997; 272(13):8133-6. [PMID: 9079625]
- Lim S, Longley RL, Couchman JR, and Woods A. Direct binding of syndecan-4 cytoplasmic domain to the catalytic domain of protein kinase C alpha (PKC alpha) increases focal adhesion localization of PKC alpha. J. Biol. Chem. 2003; 278(16):13795-802. [PMID: 12571249]
- Koo B, Jung YS, Shin J, Han I, Mortier E, Zimmermann P, Whiteford JR, Couchman JR, Oh E, and Lee W. Structural basis of syndecan-4 phosphorylation as a molecular switch to regulate signaling. J. Mol. Biol. 2005; 355(4):651-63. [PMID: 16310216]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, and Luo L. Rac GTPases control axon growth, guidance and branching. Nature 2002; 416(6879):442-7. [PMID: 11919635]
- Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, and Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell. Sci. 2000; 113 ( Pt 20):3673-8. [PMID: 11017882]
- Schober M, Raghavan S, Nikolova M, Polak L, Pasolli HA, Beggs HE, Reichardt LF, and Fuchs E. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 2007; 176(5):667-80. [PMID: 17325207]
- Arthur WT, and Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell 2001; 12(9):2711-20. [PMID: 11553710]
- Tomar A, Lim S, Lim Y, and Schlaepfer DD. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J. Cell. Sci. 2009; 122(Pt 11):1852-62. [PMID: 19435801]
- Bradley WD, Hernández SE, Settleman J, and Koleske AJ. Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120RasGAP and recruitment to the membrane. Mol. Biol. Cell 2006; 17(11):4827-36. [PMID: 16971514]
- Brouns MR, Matheson SF, Hu KQ, Delalle I, Caviness VS, Silver J, Bronson RT, and Settleman J. The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development 2000; 127(22):4891-903. [PMID: 11044403]
- Bass MD, Morgan MR, Roach KA, Settleman J, Goryachev AB, and Humphries MJ. p190RhoGAP is the convergence point of adhesion signals from alpha 5 beta 1 integrin and syndecan-4. J. Cell Biol. 2008; 181(6):1013-26. [PMID: 18541700]
- Vicente-Manzanares M, Newell-Litwa K, Bachir AI, Whitmore LA, and Horwitz AR. Myosin IIA/IIB restrict adhesive and protrusive signaling to generate front-back polarity in migrating cells. J. Cell Biol. 2011; 193(2):381-96. [PMID: 21482721]
- Iwanicki MP, Vomastek T, Tilghman RW, Martin KH, Banerjee J, Wedegaertner PB, and Parsons JT. FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J. Cell. Sci. 2008; 121(Pt 6):895-905. [PMID: 18303050]
- Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, and Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 2011; 13(6):722-7. [PMID: 21572419]
- Dovas A, Yoneda A, and Couchman JR. PKCbeta-dependent activation of RhoA by syndecan-4 during focal adhesion formation. J. Cell. Sci. 2006; 119(Pt 13):2837-46. [PMID: 16787950]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, and Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 1996; 271(34):20246-9. [PMID: 8702756]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, and Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science 1996; 273(5272):245-8. [PMID: 8662509]
- Le Clainche C, and Carlier M. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 2008; 88(2):489-513. [PMID: 18391171]
- Zaidel-Bar R, Kam Z, and Geiger B. Polarized downregulation of the paxillin-p130CAS-Rac1 pathway induced by shear flow. J. Cell. Sci. 2005; 118(Pt 17):3997-4007. [PMID: 16129884]
- Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, and Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell 2008; 135(3):510-23. [PMID: 18984162]
- Lamarche-Vane N, and Hall A. CdGAP, a novel proline-rich GTPase-activating protein for Cdc42 and Rac. J. Biol. Chem. 1998; 273(44):29172-7. [PMID: 9786927]


