What is the role of cortactin in actin polymerization?
Cortactin is a class II nucleation promoting factor (NPF) that binds to actin filaments and influences their stability. Cortactin specifically stabilizes Arp2/3-mediated branch points along actin filaments through its repetitive actin binding sites [1] (reviewed in [2]). Although cortactin is a weak activator of the Arp2/3 complex when compared to class I NPFs (e.g. WASP, SCAR/WAVE), cortactin also binds to other NPFs (e.g. N-WASP) and their interacting proteins (e.g. WIP). This association may help to both recruit and activate Arp2/3 complex-mediated nucleation of actin filaments [3][4][5][6][7].
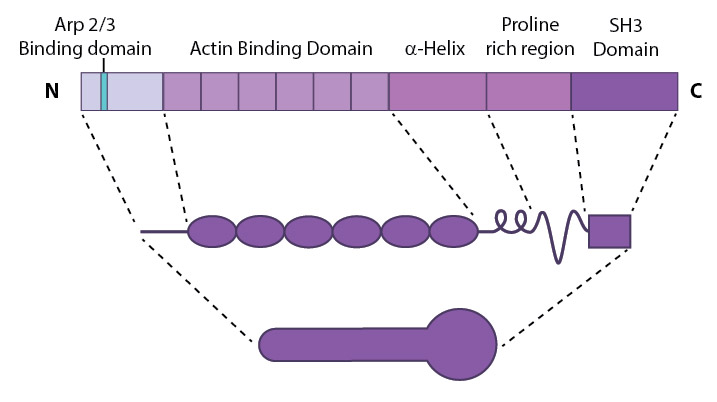
This schematic diagram illustrates the molecular organization of cortactin (reviewed in [2]) and provides examples for how cortactin is represented in figures throughout this resource. Relevant domains believed to be important for cortactin activity are highlighted.
Where else is cortactin found?
Functionally, cortactin is involved in a wide range of cellular processes pertaining to a variety of structures. The protein is highly enriched in mature neurons and is necessary for the formation and stability of dendritic spines [13]. It also associates with post synaptic densities (PSDs) of neurons expressing the ionotropic glutamate receptor, NMDA (N-methyl-D-aspartate). This association is via a weak interaction with the PSD protein Shank [10]. The function of cortactin at PSDs is not yet clear but it is speculated to facilitate changes in the actin cytoskeleton in response to synaptic activity. This is supported by the translocation of cortactin to synapses in response to stimulation by glutamate [10].
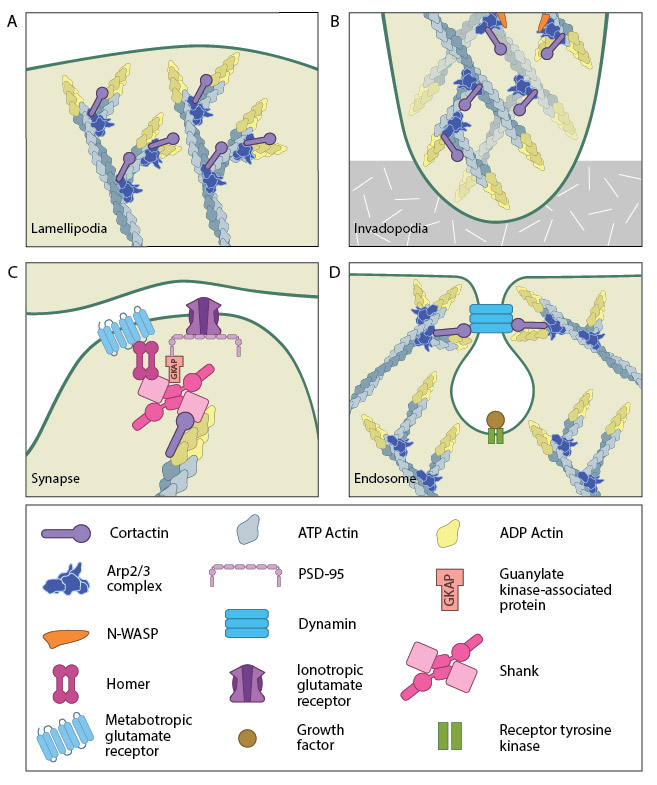
A. Cortactin stabilizes Arp2/3-mediated actin nucleation. B. Cortactin forms part of the actin-rich core of both podosomes and invadopodia. C. Cortactin is enriched in post-synaptic densities involved in glutametergic signalling. D. Cortactin binds to dynamic during endocytosis.
Cortactin also forms part of the actin-rich core of both podosomes and invadopodia [14]. Actin polymerization within invadopodia is essential to the function of this structure and the migratory behavior of cancerous cells [13]. Cortactin also promotes cell motility in normal cells and as such localizes to lamellipodia [15][16]. It enhances lamellipodial persistence i.e. increases the time spent protruding, through its interactions with both Arp2/3 and F-actin. Cortactin demonstrates a notable preference for newly polymerized actin filaments i.e. those containing a greater amount of ATP-actin over ADP-Pi-actin [15]. However cortactin itself is not thought to nucleate actin within the lamellipodium [17], but rather serves to stabilize newly polymerized actin filaments in order to promote lamellipodial persistence [15][17].
Cortactin is also involved in non-migratory cellular processes, such as the formation of cell-cell contacts. Cortactin localizes to sites of N-cadherin mediated cell-cell adhesions, where it forms a complex with N-cadherin and serves to strengthen the intercellular contacts [18]. It also localizes to endosomal compartments [16], specifically clathrin-coated pits [19], where it is involved in receptor-mediated endocytosis. It is suggested to link the actin cytoskeleton and the endocytosing vesicle through its known interactions with F-actin, dynamin-2 and Arp2/3 [19]. These interactions are speculated to not only support a role for cortactin as a scaffold, but also to contribute to the dynamic action of endocytosis through the actin nucleating activity of cortactin [16][19].
References
- Uruno T, Liu J, Zhang P, Fan Yx , Egile C, Li R, Mueller SC, and Zhan X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 2001; 3(3):259-66. [PMID: 11231575]
- Ammer AG, and Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil. Cytoskeleton 2008; 65(9):687-707. [PMID: 18615630]
- Kinley AW, Weed SA, Weaver AM, Karginov AV, Bissonette E, Cooper JA, and Parsons JT. Cortactin interacts with WIP in regulating Arp2/3 activation and membrane protrusion. Curr. Biol. 2003; 13(5):384-93. [PMID: 12620186]
- Uruno T, Liu J, Li Y, Smith N, and Zhan X. Sequential interaction of actin-related proteins 2 and 3 (Arp2/3) complex with neural Wiscott-Aldrich syndrome protein (N-WASP) and cortactin during branched actin filament network formation. J. Biol. Chem. 2003; 278(28):26086-93. [PMID: 12732638]
- Martinez-Quiles N, Ho HH, Kirschner MW, Ramesh N, and Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol. Cell. Biol. 2004; 24(12):5269-80. [PMID: 15169891]
- Kowalski JR, Egile C, Gil S, Snapper SB, Li R, and Thomas SM. Cortactin regulates cell migration through activation of N-WASP. J. Cell. Sci. 2004; 118(Pt 1):79-87. [PMID: 15585574]
- Tehrani S, Tomasevic N, Weed S, Sakowicz R, and Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc. Natl. Acad. Sci. U.S.A. 2007; 104(29):11933-8. [PMID: 17606906]
- Buccione R, Caldieri G, and Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009; 28(1-2):137-49. [PMID: 19153671]
- Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, Cooper JA, and Parsons JT. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 2000; 151(1):29-40. [PMID: 11018051]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, and Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 1999; 23(3):569-82. [PMID: 10433268]
- McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, and Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell Biol. 2000; 151(1):187-98. [PMID: 11018064]
- Mizutani K, Miki H, He H, Maruta H, and Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002; 62(3):669-74. [PMID: 11830518]
- Mader CC, Oser M, Magalhaes MAO, Bravo-Cordero JJ, Condeelis J, Koleske AJ, and Gil-Henn H. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011; 71(5):1730-41. [PMID: 21257711]
- Destaing O, Saltel F, Géminard J, Jurdic P, and Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell 2003; 14(2):407-16. [PMID: 12589043]
- Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, and Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr. Biol. 2005; 15(14):1276-85. [PMID: 16051170]
- Kaksonen M, Peng HB, and Rauvala H. Association of cortactin with dynamic actin in lamellipodia and on endosomal vesicles. J. Cell. Sci. 2000; 113 Pt 24:4421-6. [PMID: 11082035]
- Lai FPL, Szczodrak M, Block J, Faix J, Breitsprecher D, Mannherz HG, Stradal TEB, Dunn GA, Small JV, and Rottner K. Arp2/3 complex interactions and actin network turnover in lamellipodia. EMBO J. 2008; 27(7):982-92. [PMID: 18309290]
- El Sayegh TY, Arora PD, Laschinger CA, Lee W, Morrison C, Overall CM, Kapus A, and McCulloch CAG. Cortactin associates with N-cadherin adhesions and mediates intercellular adhesion strengthening in fibroblasts. J. Cell. Sci. 2004; 117(Pt 21):5117-31. [PMID: 15383621]
- Cao H, Orth JD, Chen J, Weller SG, Heuser JE, and McNiven MA. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol. Cell. Biol. 2003; 23(6):2162-70. [PMID: 12612086]


