What is the role of cadherin and nectin recruitment in adherens junction assembly?
Cadherin Recruitment
Type I cadherins, also known as classical cadherins, are commonly found in adherens junctions (AJs) and desmosomes. They are produced as a precursor polypeptide that is non-adhesive. This pro-form must be proteolytically cleaved at its N terminus to produce a functional cadherin, after which the cadherin is trafficked to the plasma membrane for use [1]. Cadherins comprise five extracellular cadherin (EC) repeats in their ectodomain. These highly conserved repeats are a hallmark of the cadherin superfamily and are roughly 110 amino acids in length. Each EC domain comprises seven β-strands (labeled A to G) arranged into two β-sheets, with opposite orientations to facilitate tandem repeating of these subdomains [2][3]. The desmosomal cadherins, desmoglein and desmocollin, also follow this pattern and contain five EC repeats [4]
It is currently unknown precisely how the cadherin-catenin complex is recruited to sites of cell-cell contact, with several different models being posited including, but not limited to; nectin-based recruitment and spontaneous recruitment.
Nectin-based Recruitment
Evidence from epithelial cell culture suggests that the establishment of early nectin-nectin based adhesions leads to the recruitment of cadherins, although the requirement for nectin-based interactions in AJ formation in vivo is far less clear (as reviewed in [5]). In cell culture scenarios various proteins are suggested to bridge the gap between the nectin-afadin complex and the cadherin-catenin complex.
During early junction formation vinculin and ponsin are suggested to link the nectins to the cadherins. Vinculin is known to bind α-catenin that can bind β-catenin, which directly interacts with cadherin. In vitro experiments show ponsin is able to bind both afadin and vinculin, however these interactions are competitive, suggesting additional proteins are needed in vivo to allow ponsin and vinculin to recruit cadherins to nectin-based adhesion sites [6].
Other protein complexes connecting nectin-afadin to cadherin-catenin include LMO7–α-actinin [7] and ADIP-α-actinin [8]. However it is unclear at what point during junction formation these proteins come into play, with evidence suggesting LMO7 is only involved at a more mature stage of junction development [7].
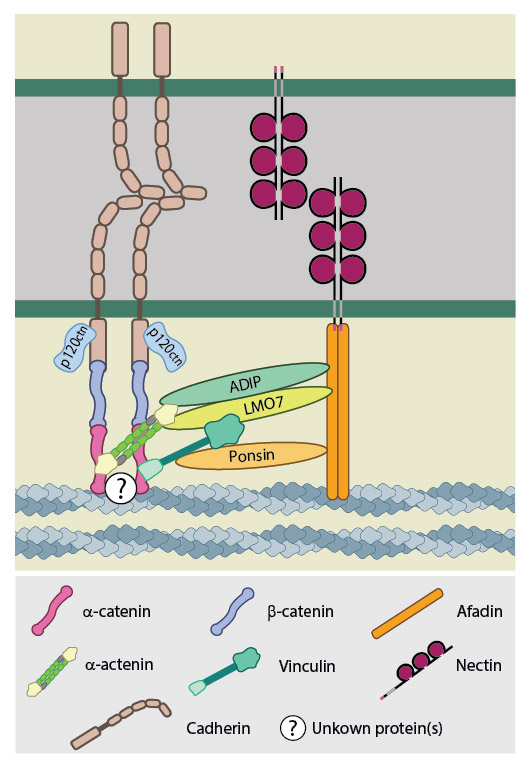
Nectin-based adhesions recruit cadherins to form the adherens junction. These receptors are indirectly linked through interactions at the cytoplasmic face. The proteins connecting the cadherin-catenin and nectin-afadin complexes are not known in their entirety, though several have been posited including; ponsin-vinculin, ADIP-α-actinin and LMO7-α-actinin. The latter complex is suggested to be involved in more mature adherens junctions and is therefore unlikely to be involved in the initial recruitment of cadherin by nectin [ref]. The activities ofRho GTPases, such as Rac and Cdc42, also have a role to play in cadherin recruitment through their downstream effectors. These effectors include IQGAP1, which is recruited to early adhesion sites by nectins [ref] and is suggested to aid in the recruitment of cadherin-catenin complexes to nectin-based adhesion sites by preventing the endocytosis of cell surface cadherins.
- Ozawa M, and Kemler R. Correct proteolytic cleavage is required for the cell adhesive function of uvomorulin. J. Cell Biol. 1990; 111(4):1645-50. [PMID: 2211831]
- Overduin M, Harvey TS, Bagby S, Tong KI, Yau P, Takeichi M, and Ikura M. Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science 1995; 267(5196):386-9. [PMID: 7824937]
- Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grübel G, Legrand JF, Als-Nielsen J, Colman DR, and Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature 1995; 374(6520):327-37. [PMID: 7885471]
- Buxton RS, and Magee AI. Structure and interactions of desmosomal and other cadherins. Semin. Cell Biol. 1992; 3(3):157-67. [PMID: 1623205]
- Harris TJC, and Tepass U. Adherens junctions: from molecules to morphogenesis. Nat. Rev. Mol. Cell Biol. 2010; 11(7):502-14. [PMID: 20571587]
- Mandai K, Nakanishi H, Satoh A, Takahashi K, Satoh K, Nishioka H, Mizoguchi A, and Takai Y. Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell-cell and cell-matrix adherens junctions. J. Cell Biol. 1999; 144(5):1001-17. [PMID: 10085297]
- Ooshio T, Irie K, Morimoto K, Fukuhara A, Imai T, and Takai Y. Involvement of LMO7 in the association of two cell-cell adhesion molecules, nectin and E-cadherin, through afadin and alpha-actinin in epithelial cells. J. Biol. Chem. 2004; 279(30):31365-73. [PMID: 15140894]
- Asada M, Irie K, Morimoto K, Yamada A, Ikeda W, Takeuchi M, and Takai Y. ADIP, a novel Afadin- and alpha-actinin-binding protein localized at cell-cell adherens junctions. J. Biol. Chem. 2002; 278(6):4103-11. [PMID: 12446711]


