What is the role of adaptor proteins in the stabilization and function of core adherens junction components?
Recruitment of plaque proteins to adherens junctions
The difficulties in establishing a direct link between the α-catenin–β-catenin complex and the actin cytoskeleton underlies a higher degree of complexity in the intracellular structure of AJs. Much of this complexity stems from the multiple interactions that each component can engage. For example, p120-catenin controls cadherin’s endocytosis however it has also been shown to recruit additional adaptor proteins including afadin, and in turn ZO-1, as well as cortactin [1][2][3][4]. Depending on the cell type and its localization, (in the nucleus or in the cytoplasm) p120-catenin has also been correlated with Rho A recruitment, AJ regulation and subsequently cancer cell invasiveness (reviewed in [5]).
To date numerous adaptor proteins have been identified within AJ sites. These proteins may be recruited to the plaque region where they function in the structural integrity of the AJ or in the regulation of complex dynamics. Vinculin, which is also found in integrin based cell-matrix adhesions, is found in AJs in association with α-catenin where it has been proposed to bind to a novel binding site that is thought to be exposed only when α-catenin has been stretched [6]. Like vinculin, afadin, which is recruited to the AJ by p120-catenin [7][8], α-actinin and EPLIN can all bind α-catenin and the actin filament network [9][10]. Fitting with the complex and dynamic nature of interactions within the AJ, it has been reported that vinculin, as well as afadin do not bind to the C-terminal domain of α-catenin [11][12][8] which was highlighted as being essential in the rescue of cadherin function in fibroblasts [13].
Scaffolding functions of Eplin at adherens junctions
Recruitment of EPLIN, which is not expressed in all cell types but is found exclusively in AJ, has also been shown to be tension dependent [14][15][16]. In this case EPLIN binds to the sides of F-actin where it stabilizes and/or crosslinks bundles of actin filaments to prevent Arp2/3 binding and subsequently, filament branching [14]. Whilst serving this role, EPLIN may also interact with α-catenin in complex with cadherin and stabilize actin filament bundles that span from one AJ to the next (i.e. the adhesion belt) [15]. In vitro experiments have shown that whilst monomers of α-catenin could not bind actin filaments, EPLIN could. Moreover, depletion of EPLIN from epithelial cells resulted in disruption of the adhesion belt, with the cell-cell contacts taking on a more immature appearance i.e. radial actin filaments terminating at puncta of E-cadherin present at contact sites [15]. Collectively these studies [14][15] suggest EPLIN may promote AJ maturation by linking the catenin-cadherin complex to the actin cytoskeleton and promoting adhesion belt formation and stabilization. Again, in certain situations the other proteins can compensate for this adaptor protein, as shown by one study that reported normally formed actin bundles and zonula adhesions despite the introduction of an EPLIN binding site deficient mutant of α-catenin in epithelial cells that otherwise lacked functional α-catenin [6]
Importantly, adaptor proteins localized at AJs may also facilitate the recruitment of other proteins that function in associated mechanisms such as cytoskeletal organization. It is known for example that AJ adaptor proteins recruit the Arp2/3 complex, formins and other proteins involved in the regulation of the actin cytoskeleton [17][18][19]. Overall, the complex and highly dynamic arrangement of the AJ may enable it to endure the transient yet strong forces produced during processes such as apical constriction, where the actin cytoskeleton pulls upon the adhesion complex.
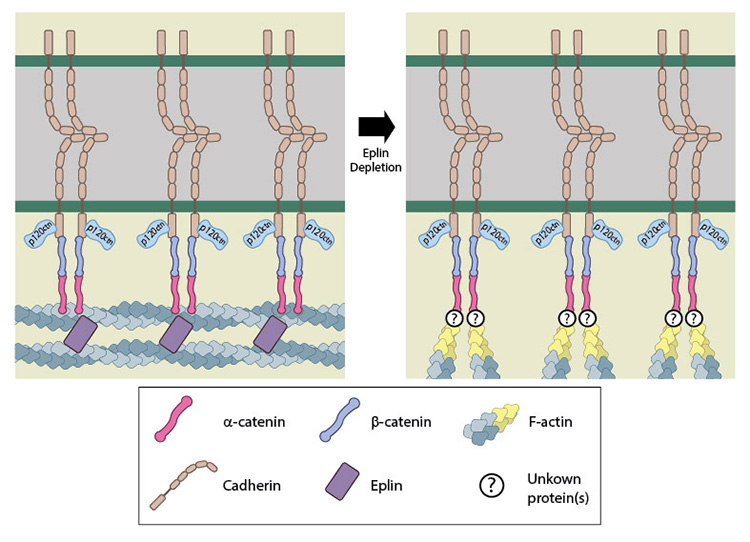
EPLIN is able to bind α-catenin, bound to the β-catenin-cadherin complex, as well as actin. It is therefore suggested as a candidate for linking the cadherin-catenin complex to the actin cytoskeleton. Moreover, it is also suggested to promote and stablize formation of the adhesion belt, with its loss causing the actin cytoskeleton in this region to take on a more immature appearance of radial filaments.
Transient linkages at adherens junctions
It is unlikely that any single protein is responsible for linking the catenin-cadherin complex with the actin cytoskeleton. Instead, eplin and vinculin, as well as additional candidate proteins such as ZO-1, α-actinin, afadin [20] are likely to contribute in a manner that is cell type dependent. The interactions that facilitate this link are also likely to be transient which is consistent with both adaptor protein turnover rate, which is within minutes, and the dynamic nature of the cortical actin cytoskeleton as revealed by FRAP experiments [21]. Whilst this makes detection of any link difficult using current experimental techniques it should be noted that even numerous weak, transient interactions could still promote strong linkage between cadherins and the actin cytoskeleton through sheer numbers (as reviewed in [22]. These interactions may be cell type specific and given their importance in maintaining tissue integrity may also exhibit protein redundancy.
References
- Sato T, Fujita N, Yamada A, Ooshio T, Okamoto R, Irie K, and Takai Y. Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 2005; 281(8):5288-99. [PMID: 16361708]
- Yokoyama S, Tachibana K, Nakanishi H, Yamamoto Y, Irie K, Mandai K, Nagafuchi A, Monden M, and Takai Y. alpha-catenin-independent recruitment of ZO-1 to nectin-based cell-cell adhesion sites through afadin. Mol. Biol. Cell 2001; 12(6):1595-609. [PMID: 11408571]
- Birukova AA, Fu P, Wu T, Dubrovskyi O, Sarich N, Poroyko V, and Birukov KG. Afadin controls p120-catenin-ZO-1 interactions leading to endothelial barrier enhancement by oxidized phospholipids. J. Cell. Physiol. 2012; 227(5):1883-90. [PMID: 21732359]
- Boguslavsky S, Grosheva I, Landau E, Shtutman M, Cohen M, Arnold K, Feinstein E, Geiger B, and Bershadsky A. p120 catenin regulates lamellipodial dynamics and cell adhesion in cooperation with cortactin. Proc. Natl. Acad. Sci. U.S.A. 2007; 104(26):10882-7. [PMID: 17576929]
- Menke A, and Giehl K. Regulation of adherens junctions by Rho GTPases and p120-catenin. Arch. Biochem. Biophys. 2012; 524(1):48-55. [PMID: 22583808]
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, and Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 2010; 12(6):533-42. [PMID: 20453849]
- Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, and Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J. Cell Biol. 2000; 150(5):1161-76. [PMID: 10974003]
- Pokutta S, Drees F, Takai Y, Nelson WJ, and Weis WI. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J. Biol. Chem. 2002; 277(21):18868-74. [PMID: 11907041]
- Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR, and Wheelock MJ. Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J. Cell. Sci. 1997; 110 ( Pt 8):1013-22. [PMID: 9152027]
- Chervin-Pétinot A, Courçon M, Almagro S, Nicolas A, Grichine A, Grunwald D, Prandini M, Huber P, and Gulino-Debrac D. Epithelial protein lost in neoplasm (EPLIN) interacts with α-catenin and actin filaments in endothelial cells and stabilizes vascular capillary network in vitro. J. Biol. Chem. 2011; 287(10):7556-72. [PMID: 22194609]
- Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson ED, and Takeichi M. alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J. Cell Biol. 1998; 142(3):847-57. [PMID: 9700171]
- Weiss EE, Kroemker M, Rüdiger AH, Jockusch BM, and Rüdiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between alpha-catenin and vinculin. J. Cell Biol. 1998; 141(3):755-64. [PMID: 9566974]
- Imamura Y, Itoh M, Maeno Y, Tsukita S, and Nagafuchi A. Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J. Cell Biol. 1999; 144(6):1311-22. [PMID: 10087272]
- Maul RS, Song Y, Amann KJ, Gerbin SC, Pollard TD, and Chang DD. EPLIN regulates actin dynamics by cross-linking and stabilizing filaments. J. Cell Biol. 2003; 160(3):399-407. [PMID: 12566430]
- Abe K, and Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc. Natl. Acad. Sci. U.S.A. 2007; 105(1):13-9. [PMID: 18093941]
- Taguchi K, Ishiuchi T, and Takeichi M. Mechanosensitive EPLIN-dependent remodeling of adherens junctions regulates epithelial reshaping. J. Cell Biol. 2011; 194(4):643-56. [PMID: 21844208]
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, and Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr. Biol. 2002; 12(5):379-82. [PMID: 11882288]
- Kobielak A, Pasolli HA, and Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat. Cell Biol. 2003; 6(1):21-30. [PMID: 14647292]
- Helwani FM, Kovacs EM, Paterson AD, Verma S, Ali RG, Fanning AS, Weed SA, and Yap AS. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J. Cell Biol. 2004; 164(6):899-910. [PMID: 15024035]
- Yonemura S. Cadherin-actin interactions at adherens junctions. Curr. Opin. Cell Biol. 2011; 23(5):515-22. [PMID: 21807490]
- Yamada S, Pokutta S, Drees F, Weis WI, and Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell 2005; 123(5):889-901. [PMID: 16325582]
- Gates J, and Peifer M. Can 1000 reviews be wrong? Actin, alpha-Catenin, and adherens junctions. Cell 2005; 123(5):769-72. [PMID: 16325573]


