What is the mesenchymal-epithelial transition?
During development, the transitioning of epithelial cells to a mesenchymal lineage is not an irreversible process. Instead, a number of developmental programs are accompanied by a reversal of EMT, during which the mesenchyme gets dedifferentiated into the epithelial lineage through a series of molecular events, known as mesenchymal to epithelial transition (MET). This involves morphological changes such as cell polarization and establishment of cell-cell contacts, which are accompanied by changes in the activities of several transcriptional factors that regulate the expression levels of epithelial vs mesenchymal cell markers and signaling molecules. As well as its occurrence during embryonic developmental programs, MET has been observed during reprogramming of somatic cells into induced pluripotent stem cells and in disease states such as fibrosis, inflammation, and cancers [1]. Armed with an enhanced understanding of the molecular basis of MET, recent research in the field of regenerative medicine has exploited MET as the chief intermediary step in the generation of induced pluripotent stem cells (iPSCs) from mesenchymal cells [2][3].
Although most organs develop from epithelial tissue during embryogenesis, there are also instances when certain tissue, such as the epithelia of the genitourinary system, peritoneal membranes, and Drosophila midgut are derived from mesenchymal tissue [4]. The development of the mammalian kidney tubules is a classic example of a morphogenetic event that involves the conversion of metanephric mesenchymal cells into epithelial cells that line the nephrons [5][6]. The mammalian nephric system is one of the most complex organ systems to be formed during embryogenesis and involves the interdependent development of two major components: the ureteric bud and the metanephric mesenchyme. The formation of the adult kidney starts off with the sprouting up of a pair of epithelial buds, called ureteric buds, from the nephric or the Wolffian duct in response to signals from the metanephric mesenchyme. The ureteric buds proliferate and frequently branch-off, evolving into a complex network of tubules called the collecting ducts that invade into the metanephric mesenchyme. During this process of extensive branching, the metanephric mesenchyme responds to reciprocal inductive signals from the ureteric bud by condensing at the tips of ureteric branches, and start expressing epithelial cell markers such as cadherins and extracellular matrix proteins such as collagen IV and laminin. These epithelial cells derived from MET form structures called renal vesicles that ultimately fuse with the ureteric ducts to form the functional nephrons. Studies have linked failure of MET during kidney development with the occurrence of Wilm’s tumor, a malignancy of kidney cells [7][4].
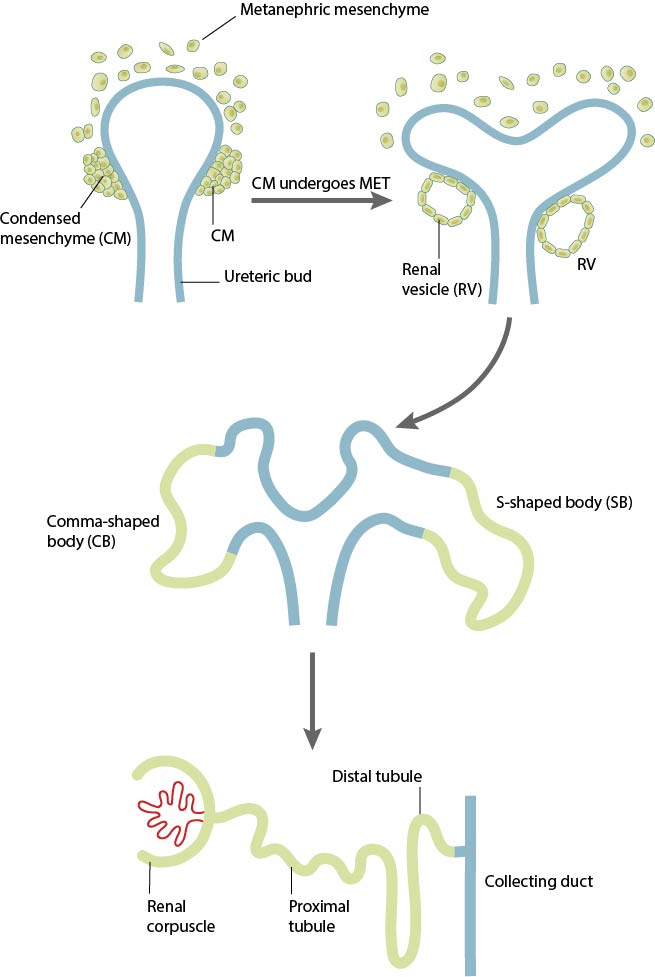
In response to signals arising from the ureteric bud, the metanephric mesenchyme (MM) surrounding the UB condense around the tips of nascent ureteric branches to form the cap mesemchyme (CM). The CM, which is observed to a self-renewing progenitor cell population, starts undergoing a progressive mesenchymal-epithelial transition (MET) that leads to the formation of an epithelial renal vesicle (RV). THE RVs are the precursors to functional nephrons, as they twist and elongate into tightly patterned and segmented tubules (forming comma-shaped and S-shaped intermediary bodies) that remain fused with the ureteric branches to form contiguous tubules with the collecting ducts.
MET during nephrogenesis is regulated by signals from the surrounding tissues
As detailed above, coordinated, reciprocal inductive interactions between the ureteric bud and metanephric mesenchyme are needed for cell specification into a nephric lineage and early kidney development. For example, the formation of the ureteric bud and its branching morphology is spatially and temporally regulated by signals arising from the metanephric mesenchyme. However, differentiation and survival of the mesenchymal cells as well as their conversion into an epithelial lineage is controlled by inductive signals, such as growth factors and Wnt ligands that are secreted by the ureteric bud. Studies using mice embryos have demonstrated a role for Wnt9b, which is expressed on the stalk of the ureteric bud, in regulating mesenchymal-epithelial transition and the induction of renal vesicles by activating transcription factors such as Pax8 and downstream wnt targets such as Wnt4 in the metanephric mesenchyme [8]. In addition to this reciprocal signaling between the ureteric bud and the mesenchyme, certain growth factors secreted by the ectoderm, such as bone morphogenetic protein-4 (BMP-4), have been shown to induce the expression of the earliest kidney specific markers, the transcription factors Pax-2 and Pax-4, in the intermediate mesoderm during mouse, frog, and zebrafish development. The intermediate mesodermal cells of mutant mouse embryos lacking both Pax-2 and Pax-8 expression failed to convert into an epithelial lineage that is essential for the formation of functional nephrons, and instead, underwent apoptosis at later time points [9][10].
Mechano-regulation of mesenchymal-epithelial transition during development
The transitioning of cells from a mesenchymal to an epithelial lineage is marked by changes in cell polarity, cell motility, cell-cell contact formation, cytoskeletal organization, and extracellular matrix composition. The expression of E-cadherin is upregulated in the epithelial phenotype, which mediates the formation of stable cell-cell contacts and maintains tissue integrity. In studies using mice and human somatic cells, exogenous E-cadherin expression was sufficient to overcome the requirement for the transcription factor Oct4 in the reprogramming of mouse fibroblasts into pluripotent stem cells [11], while depletion of E-cadherin led to impaired mesenchymal-epithelial transition in human somatic cells [12]. In the developing mammalian kidney, type II cadherins such as cadherin-6, cadherin-11, and R-cadherin are also known to be dynamically expressed and play a vital role in regulating the conversion of the metanephric mesenchyme into an epithelial lineage. For instance, mutant mice embryos lacking cadherin-6 exhibited a delay in the conversion of metanephric mesenchyme to the epithelial phenotype, as well as delayed or disrupted fusion of the renal vesicle with the ureteric bud. This led to a number of non-functional nephrons that subsequently underwent necrosis during development, resulting in a decline in the nephron populations within the kidneys of adult mice [13].
In addition to biochemical signaling, biophysical signals such as substrate topography and other mechanical properties of the cellular microenvironment also have a direct impact on the reprogramming ability of somatic cells. Growing cells on micro- and nano-patterned bioengineered substrates can introduce changes in actomyosin contractility, which can modify the cells’ epigenetic state by increasing histone acetylation and methylation levels in genes encoding for epithelial markers. This has led to improved efficiency in reprogramming somatic cells into induced pluripotent stem cells [14]. A number of studies have also highlighted the regulatory effects of the Rho family of small GTPases, which are key modulators of cytoskeletal organization in response to mechanical signals, during mesenchymal-epithelial transitions. Maintaining correct levels of Rac1 is necessary to promote an epithelial lineage, while activated Cdc42 and RhoA led to cells adopting a mesenchymal lineage [15][16].
References
- Demirkan B. The Roles of Epithelial-to-Mesenchymal Transition (EMT) and Mesenchymal-to-Epithelial Transition (MET) in Breast Cancer Bone Metastasis: Potential Targets for Prevention and Treatment. J Clin Med 2013; 2(4):264-82. [PMID: 26237148]
- Yang J, and Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 2008; 14(6):818-29. [PMID: 18539112]
- Li R, Liang J, Ni S, Zhou T, Qing X, Li H, He W, Chen J, Li F, Zhuang Q, Qin B, Xu J, Li W, Yang J, Gan Y, Qin D, Feng S, Song H, Yang D, Zhang B, Zeng L, Lai L, Esteban MA, and Pei D. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell 2010; 7(1):51-63. [PMID: 20621050]
- Mori K, Yang J, and Barasch J. Ureteric bud controls multiple steps in the conversion of mesenchyme to epithelia. Semin. Cell Dev. Biol. 2003; 14(4):209-16. [PMID: 14627119]
- Saxén L, and Sariola H. Early organogenesis of the kidney. Pediatr. Nephrol. 1987; 1(3):385-92. [PMID: 3153305]
- Ekblom P. Developmentally regulated conversion of mesenchyme to epithelium. FASEB J. 1989; 3(10):2141-50. [PMID: 2666230]
- Little M, Georgas K, Pennisi D, and Wilkinson L. Kidney development: two tales of tubulogenesis. Curr. Top. Dev. Biol. 2010; 90:193-229. [PMID: 20691850]
- Carroll TJ, Park J, Hayashi S, Majumdar A, and McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 2005; 9(2):283-92. [PMID: 16054034]
- Bouchard M, Souabni A, Mandler M, Neubüser A, and Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002; 16(22):2958-70. [PMID: 12435636]
- Torres M, Gómez-Pardo E, Dressler GR, and Gruss P. Pax-2 controls multiple steps of urogenital development. Development 1995; 121(12):4057-65. [PMID: 8575306]
- Redmer T, Diecke S, Grigoryan T, Quiroga-Negreira A, Birchmeier W, and Besser D. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep. 2011; 12(7):720-6. [PMID: 21617704]
- Li D, Zhou J, Wang L, Shin ME, Su P, Lei X, Kuang H, Guo W, Yang H, Cheng L, Tanaka TS, Leckband DE, Reynolds AB, Duan E, and Wang F. Integrated biochemical and mechanical signals regulate multifaceted human embryonic stem cell functions. J. Cell Biol. 2010; 191(3):631-44. [PMID: 20974810]
- Mah SP, Saueressig H, Goulding M, Kintner C, and Dressler GR. Kidney development in cadherin-6 mutants: delayed mesenchyme-to-epithelial conversion and loss of nephrons. Dev. Biol. 2000; 223(1):38-53. [PMID: 10864459]
- Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, Chu J, Patel S, Schaffer DV, and Li S. Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater 2013; 12(12):1154-62. [PMID: 24141451]
- Shankar J, and Nabi IR. Actin cytoskeleton regulation of epithelial mesenchymal transition in metastatic cancer cells. PLoS ONE 2015; 10(3):e0119954. [PMID: 25756282]
- Nakaya Y, Kuroda S, Katagiri YT, Kaibuchi K, and Takahashi Y. Mesenchymal-epithelial transition during somitic segmentation is regulated by differential roles of Cdc42 and Rac1. Dev. Cell 2004; 7(3):425-38. [PMID: 15363416]


