What is the Hippo-YAP/TAZ tumor-suppressor pathway?
Mechanical cues control Hippo-YAP/TAZ tumor-suppressor pathway
The Hippo signaling pathway is a complex network of proteins that controls organ size via regulation of cellular proliferation, survival and differentiation. Initially discovered by genetic mosaic screens in Drosophila, the core of the Hippo pathway is comprised of two highly conserved kinases centering on the mammalian effector proteins Yes-associated protein (YAP) and its paralogue Transcriptional co-activator with PDZ-binding motif (TAZ, also known as WWTR1) [1][2][3] (Figure 1). YAP and TAZ are transcriptional co-activators that associate with several DNA-binding proteins to drive gene transcription.
Triggered by various upstream stimuli e.g. cell-cell contact [4], the MST1/2 kinases together with SAV (WW45) phosphorylate and activate LATS1/2 kinases [5][6]. Activated LATS phosphorylates YAP and TAZ on specific serine residues, particularly S127 and S89 in YAP and TAZ respectively [2][7]. Phosphorylation of these residues generates a 14-3-3-protein binding site resulting in cytoplasmic sequestration [8][9], and also marks them for degradation by the proteasome [10][11], thus inhibiting YAP and TAZ activity.
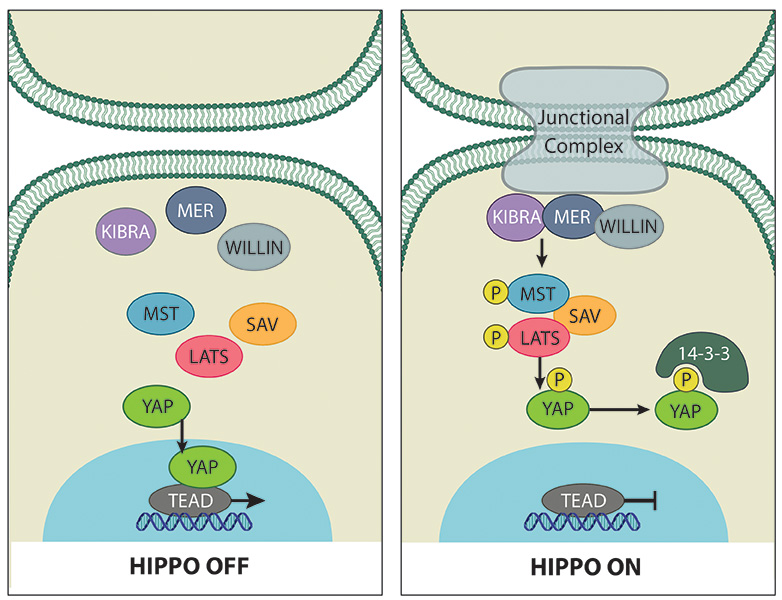
Cell to cell contact triggers the Hippo tumor suppressor pathway that controls the localization of the main effector of the pathway, YAP proto-oncogene (as well as its paralogue protein TAZ) in the cytoplasm (right panel). In sparsely populated cells YAP and TAZ translocate to the nucleus to drive transcription of proliferative genes as well as genes that inhibit apoptosis (left panel).
Mechanisms coupling extracellular signals with the core Hippo kinase cassette are complex and not yet completely understood. Recently, mechanical cues from the cytoskeleton including cell density, stretching, substrate stiffness, cellular tension, and G-protein coupled receptor signaling have been identified as regulators of YAP/TAZ activity (for comprehensive review see [12][13]) (Figure 2). Notably, mechanical signal regulation of YAP/TAZ can act either dependently or independently of the canonical Hippo pathway. The canonical Hippo pathway comprises MST (Hippo kinase ortholog in Drosophila) and LATS kinases.
Early indications that YAP/TAZ activity is regulated by mechanical cues came from the important observation that YAP localisation and phosphorylation status is regulated by cellular density [4]. In sparsely populated cells YAP is predominantly localised to the nucleus and in its ‘active’ un-phosphorylated form. In contrast in high density cell culture YAP is phosphorylated and localised to the cytoplasm, and this process is regulated by Hippo pathway signaling [4].
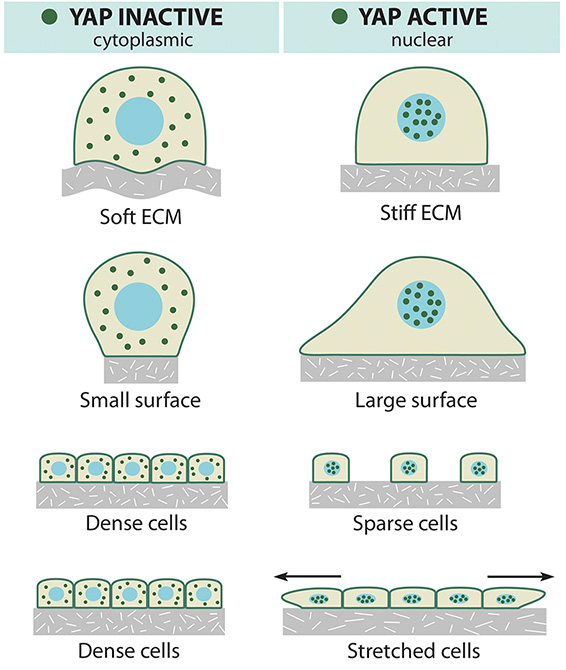
Mechanical and topological cues regulate the localization of the YAP effector protein.
Similarly, analysis of YAP/TAZ localisation and activity within a cell monolayer [14] or three-dimensional epithelial sheet [15] demonstrated that the rigidity of the extracellular matrix (ECM) and cellular geometry can regulate YAP/TAZ in a Hippo-independent manner via Rho GTPase activity and tension of the actomyosin cytoskeleton [14]. Further, sites of high mechanical stress within the 3D architecture have increased YAP/TAZ activity mediated by the F-actin capping and severing proteins (CapZ, Cofilin, and Gelsolin) [15]. The latter study also revealed that mechanical stretching of contact inhibited cells exhibiting cytoplasmic localised YAP is sufficient to re-induce entry of YAP/TAZ into the nucleus to stimulate cellular proliferation [15]. This observation is important for our understanding of how the ECM microenvironment can alter cell signaling, particularly relevant to tumorigenic transformation in which Hippo pathway dysregulation and changes in ECM stiffness have been profoundly implicated [16][17].
In terms of delineating the mechanism linking the ECM and YAP/TAZ regulation, F-actin has been identified as a major player. In Drosophila imaginal discs, induction of F-actin formation induced strong overgrowth via modulation of Hippo pathway, resulting in nuclear localised Yorkie (the Drosophila orthologue of YAP) [18]. This was supported by another study that used fabricated micropatterned cell adhesive areas called microdomains of various sizes to demonstrate that in cells grown on small domains, YAP was mostly cytoplasmic, whereas YAP was nuclear on large domains [19]. These findings were correlated with the presence of stress fibers (consisting of F-actin), which indicated that F-actin-regulates phosphorylation of YAP via upstream regulation of LATS. Moreover, inhibition of actin polymerization using drugs such as Latrunculin A and Cytochalasin D, could prevent nuclear accumulation of YAP/TAZ and abolish their activity their transcriptional co-activators [19].
Angiomotins (AMOTs) are known regulators of YAP/TAZ localisation and activity via Hippo-dependent [20] and independent [21] mechanisms. Recently, it has been shown that AMOTs can bind to F-actin, and in response to perturbation of the actin cytoskeleton dissociate from actin to bind and sequester YAP to the cytoplasm [22]. Activated Hippo pathway signaling further enhances this process, since phosphorylation of AMOT by LATS inhibits F-actin binding to promote YAP cytoplasmic localisation [23].
Regulation of YAP/TAZ activity by mechanical cues is multifaceted. Another mechanism linking cytoskeletal tension to regulation of Hippo pathway activity was identified involving Ajuba inhibition of Warts (the Drosophila orthologue of LATS). Apical localisation of Ajuba by binding to α-catenin is promoted by cytoskeletal tension [24]. The outcome of this is that increased tension within the cytoskeleton increases Drosophila wing growth due to increased Yorkie activity and vice versa [24]. YAP mediated mechanotransduction influences oligodendrocyte maturation and cell shape [25].
The spectrin network is one of the most recently identified regulators of YAP/TAZ activity in response to mechanical stimuli. Spectrin functions as scaffold protein at the membrane–cytoskeleton interface via crosslinking of short F-actin filaments (reviewed in [26]). Reports from three groups identified spectrin as a regulator of Yorkie/YAP in fly and mammalian cells respectively, whereby mutation or depletion of spectrin induces Yorkie-dependent cell polarity defects or tissue overgrowth in Drosophila [27][28][29]. Although one study found that dysregulation of apical spectrin alters the activity of the upstream Hippo pathway regulator Expanded [28], the consensus appears to be that the basolateral spectrin network regulates cortical actomyosin tension which subsequently regulates Yorkie/YAP/TAZ activity by an as yet unidentified mechanism.
Interestingly, in addition to being regulated by the actin cytoskeleton, the Hippo signalling pathway exhibits feedback to influence actin assembly. F-actin was found to accumulate abnormally when Hippo pathway activity was reduced or abolished, though this was independent of Yorkie activity [30]. Additionally, truncation of YAP in the medaka fish hirame (hir) mutant reduces actomyosin-mediated tissue tension via down-regulation of ARHGAP18, a Rho GTPase activating protein [31]. The reduced tissue tension leads to tissue flattening and tissue misalignment, and indicates that YAP is required for the attainment of proper three-dimensional body shape. Thus perhaps YAP is the long sought after sensor of gravity proposed nearly a century ago by D’Arcy Thompson (Thompson, D. W. (1917) On Growth and Form, Cambridge Univ. Press).
Importantly, numerous publications have identified YAP/TAZ mediated crosstalk with other notable signaling pathways including the Wnt/β-catenin and TGFβ-SMAD pathways, and G-protein coupled receptor (GPCR) signaling. YAP/TAZ have been shown to suppress Wnt target gene expression by binding the upstream regulator Dishevelled [32][33] and association with β-catenin to sequester it in the cytoplasm [34], and/or promote its degradation [35][36]. Driven by the Hippo pathway, cytoplasmic localised YAP/TAZ sequesters SMAD complexes, effectively suppressing TGFβ signaling [37][38]. Further, interaction of the Crumbs polarity complex with TAZ/YAP relays cell density information to regulate localisation of YAP/TAZ and abrogate transcriptional activity mediated by TGFβ [38]. Recently, a functional connection between GPCR signaling and regulation of YAP/TAZ activity has been elucidated [39],[40]. Intriguingly, GPCR signaling can either activate or inhibit the Hippo-YAP pathway depending on the proteins involved by targeting LATS. Given the integral relationship between the ECM, GPCRs, RhoA [41], and now YAP/TAZ signaling, these findings have significant implications for our understanding of how cells sense and respond to their environment in incredibly complex manners.
References
- Huang J, Wu S, Barrera J, Matthews K, and Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005; 122(3):421-34. [PMID: 16096061]
- Lei Q, Zhang H, Zhao B, Zha Z, Bai F, Pei X, Zhao S, Xiong Y, and Guan K. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 2008; 28(7):2426-36. [PMID: 18227151]
- Zhang J, Smolen GA, and Haber DA. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res. 2008; 68(8):2789-94. [PMID: 18413746]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai Z, and Guan K. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007; 21(21):2747-61. [PMID: 17974916]
- Chan EHY, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, and Silljé HHW. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 2005; 24(12):2076-86. [PMID: 15688006]
- Wu S, Huang J, Dong J, and Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 2003; 114(4):445-56. [PMID: 12941273]
- Oka T, Mazack V, and Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). J. Biol. Chem. 2008; 283(41):27534-46. [PMID: 18640976]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, and Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007; 130(6):1120-33. [PMID: 17889654]
- Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC, and Yaffe MB. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000; 19(24):6778-91. [PMID: 11118213]
- Liu C, Zha Z, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, Zhao S, Xiong Y, Lei Q, and Guan K. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J. Biol. Chem. 2010; 285(48):37159-69. [PMID: 20858893]
- Zhao B, Li L, Tumaneng K, Wang C, and Guan K. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010; 24(1):72-85. [PMID: 20048001]
- Gaspar P, and Tapon N. Sensing the local environment: actin architecture and Hippo signalling. Curr. Opin. Cell Biol. 2014; 31:74-83. [PMID: 25259681]
- Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, and Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014; 588(16):2663-70. [PMID: 24747426]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, and Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474(7350):179-83. [PMID: 21654799]
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, and Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013; 154(5):1047-59. [PMID: 23954413]
- Harvey KF, Zhang X, and Thomas DM. The Hippo pathway and human cancer. Nat. Rev. Cancer 2013; 13(4):246-57. [PMID: 23467301]
- Lu P, Weaver VM, and Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 2012; 196(4):395-406. [PMID: 22351925]
- Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, Tao C, Sasaki H, and Halder G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011; 30(12):2325-35. [PMID: 21556047]
- Wada K, Itoga K, Okano T, Yonemura S, and Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development 2011; 138(18):3907-14. [PMID: 21831922]
- Adler JJ, Johnson DE, Heller BL, Bringman LR, Ranahan WP, Conwell MD, Sun Y, Hudmon A, and Wells CD. Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases. Proc. Natl. Acad. Sci. U.S.A. 2013; 110(43):17368-73. [PMID: 24101513]
- Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, and Hong W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J. Biol. Chem. 2011; 286(9):7018-26. [PMID: 21224387]
- Mana-Capelli S, Paramasivam M, Dutta S, and McCollum D. Angiomotins link F-actin architecture to Hippo pathway signaling. Mol. Biol. Cell 2014; 25(10):1676-85. [PMID: 24648494]
- Dai X, She P, Chi F, Feng Y, Liu H, Jin D, Zhao Y, Guo X, Jiang D, Guan K, Zhong TP, and Zhao B. Phosphorylation of angiomotin by Lats1/2 kinases inhibits F-actin binding, cell migration, and angiogenesis. J. Biol. Chem. 2013; 288(47):34041-51. [PMID: 24106267]
- Rauskolb C, Sun S, Sun G, Pan Y, and Irvine KD. Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell 2014; 158(1):143-156. [PMID: 24995985]
- Shimizu T, Osanai Y, Tanaka KF, Abe M, Natsume R, Sakimura K, and Ikenaka K. YAP functions as a mechanotransducer in oligodendrocyte morphogenesis and maturation. Glia 2016; 65(2):360-374. [PMID: 27807898]
- Bennett V, and Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 2001; 81(3):1353-92. [PMID: 11427698]
- Deng H, Wang W, Yu J, Zheng Y, Qing Y, and Pan D. Spectrin regulates Hippo signaling by modulating cortical actomyosin activity. Elife 2015; 4:e06567. [PMID: 25826608]
- Fletcher GC, Elbediwy A, Khanal I, Ribeiro PS, Tapon N, and Thompson BJ. The Spectrin cytoskeleton regulates the Hippo signalling pathway. EMBO J. 2015; 34(7):940-54. [PMID: 25712476]
- Wong KKL, Li W, An Y, Duan Y, Li Z, Kang Y, and Yan Y. β-Spectrin regulates the hippo signaling pathway and modulates the basal actin network. J. Biol. Chem. 2015; 290(10):6397-407. [PMID: 25589787]
- Fernández BG, Gaspar P, Brás-Pereira C, Jezowska B, Rebelo SR, and Janody F. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 2011; 138(11):2337-46. [PMID: 21525075]
- Porazinski S, Wang H, Asaoka Y, Behrndt M, Miyamoto T, Morita H, Hata S, Sasaki T, Krens SFG, Osada Y, Asaka S, Momoi A, Linton S, Miesfeld JB, Link BA, Senga T, Shimizu N, Nagase H, Matsuura S, Bagby S, Kondoh H, Nishina H, Heisenberg C, and Furutani-Seiki M. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature 2015; 521(7551):217-221. [PMID: 25778702]
- Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H, Wrana JL, and Attisano L. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev. Cell 2010; 18(4):579-91. [PMID: 20412773]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, Kuo CJ, and Camargo FD. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 2012; 493(7430):106-10. [PMID: 23178811]
- Imajo M, Miyatake K, Iimura A, Miyamoto A, and Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. EMBO J. 2012; 31(5):1109-22. [PMID: 22234184]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, and Piccolo S. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 2014; 158(1):157-70. [PMID: 24976009]
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, and Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell 2012; 151(7):1443-56. [PMID: 23245942]
- Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, and Wrana JL. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. 2008; 10(7):837-48. [PMID: 18568018]
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, and Wrana JL. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev. Cell 2010; 19(6):831-44. [PMID: 21145499]
- Yu F, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai Z, and Guan K. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013; 27(11):1223-32. [PMID: 23752589]
- Yu F, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu X, Mills GB, and Guan K. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012; 150(4):780-91. [PMID: 22863277]
- Walsh CT, Stupack D, and Brown JH. G protein-coupled receptors go extracellular: RhoA integrates the integrins. Mol. Interv. 2008; 8(4):165-73. [PMID: 18829842]


