What is the Canonical Wnt Receptor Signaling Pathway?
The canonical Wnt receptor signaling pathway is a series of molecular events that are initiated by the binding of Wnt proteins to the frizzled family of receptors on the cell surface. This ultimately activates transcription factors and results in changes to the expression of target genes. β-catenin is a key protein in the Wnt receptor signaling pathway, and through its role in cell-cell adhesion complexes, an important factor in mechanotransduction.
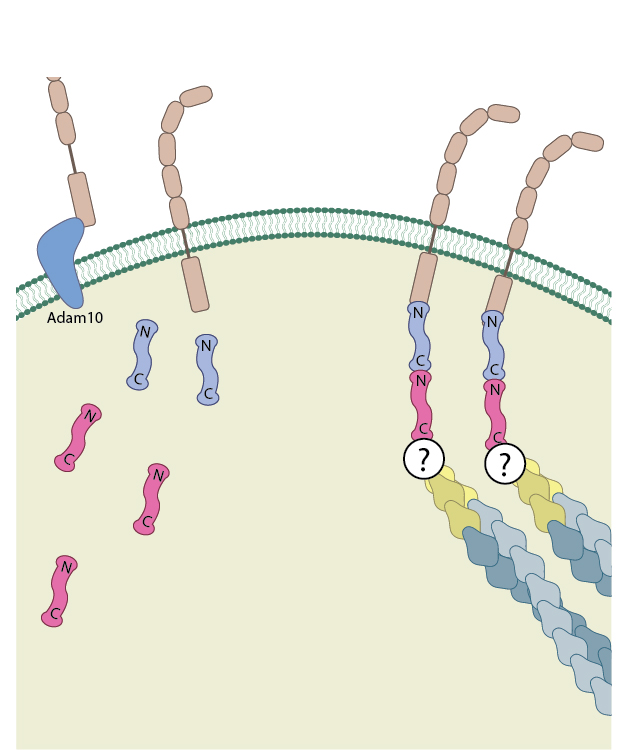
β-catenin exists as part of the adhesion complex, bound to E-cadherin and α-catenin, at the cell membrane. Disassembly of the adhesion complex by proteases such as ADAM10 releases β-catenin into the cytoplasm.
Free β-catenin is present in the cytoplasm following the disassembly of adherens junctions or cleavage of cadherins by proteases such as ADAM10 (A Disintegrin And Metalloprotease 10) [1][2]. However, due to the activity of a ‘destruction complex’, the cytoplasmic concentration of the protein is usually low. In this case free β-catenin is targeted by the scaffolding proteins APC and axin as well as the serine/threonine protein kinases CK1α which phosphorylates Ser45 and GSK3, which subsequently phosphorylate Thr41, Ser37 and Ser33 [3][4]. This phosphorylation primes the protein for degradation via the ubiquitin pathway [5]. Due to the activity of the destruction complex the majority of β-catenin exists in complex with other proteins. When bound to cadherin, an interaction that is established in the endoplasmic reticulum before release into the cytoplasm, the two proteins protect each other from degradation. Not only is β-catenin protected from interaction with components of the destruction complex, but cadherin is protected from degradation when β-catenin prevents ubiquitin ligase from binding to its PEST motif [6][7].
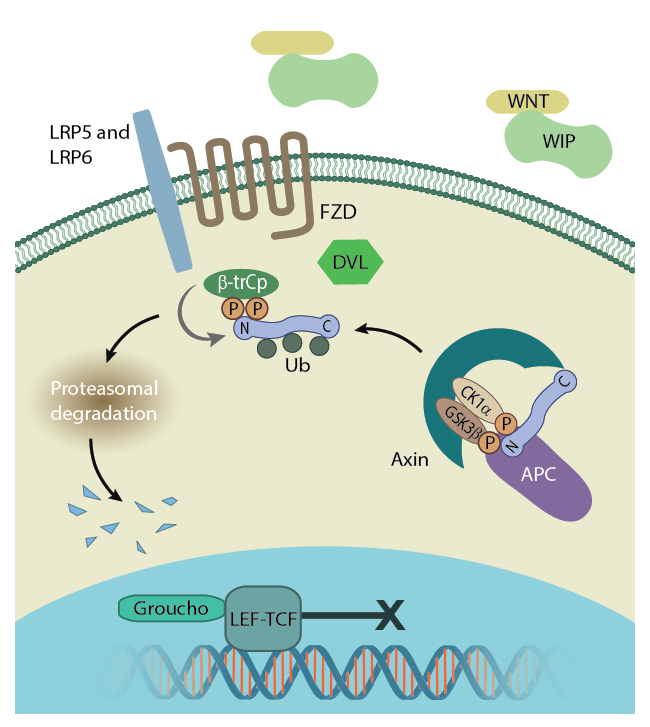
Disassembly of the adhesion complex by proteases such as ADAM10 releases β-catenin into the cytoplasm. There, and in the absence of Wnt signalling proteins, it is subject to degradation by the β-catenin destruction complex. Figure adapted from [18432252]
The exact methods by which β-catenin translocates into the nucleus remains unclear. However, it has been established that its amino acid sequence does not contain any nuclear localization signal sequence, and translocation occurs independently of classical importin/karyopherin driven nucleocytoplasmic transport. Instead, transient interactions between the ARM repeats R10-12 and components of the nuclear pore complex are believed to transport of β-catenin through the nuclear envelope [9][10].
Inside the nucleus, monomeric β-catenin forms complexes with various transcription factors to activate the transcription of various Wnt/β-catenin target genes. This is essential as β-catenin itself does not contain a DNA binding domain [11]. Helix-C, which lies after the 12 ARM repeats but before the C-terminal domain, was found to be essential for many of the interactions involved in the establishment of a transcription complex [12].
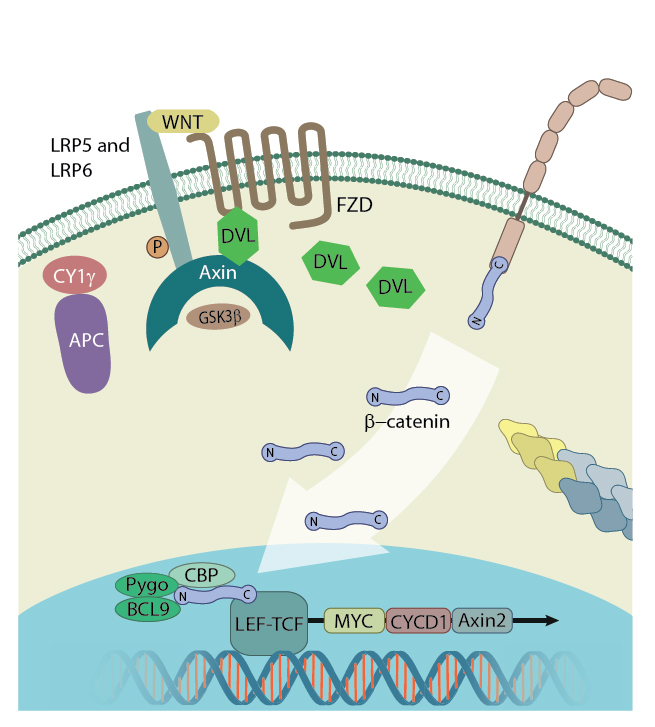
β-catenin in the nucleus: In the presence of Wnt signalling proteins, β-catenin enters the nucleus and interacts with the transcription factor LEF1 where it initiates transcription. Figure adapted from [11]
Wnt signaling in cardiac development
Intracellular signaling cascades mediated by the Wnt family of secreted signaling proteins play an essential role in regulating a number of cellular functions, such as proliferation, differentiation, adhesion, survival, apoptosis, and so on. Of their myriad signaling functions, Wnt ligands have been extensively researched for their regulatory effects on early cardiogenic events, during which the linear heart tube is transformed into a multi-chambered heart structure. Both the canonical and non-canonical Wnt signaling pathways contribute to the various stages of embryonic heart formation, confirmed by the expression of multiple Wnt genes, including canonical Wnts (Wnt1, Wnt3a, and Wnt8), and non-canonical Wnts (Wnt5a and Wnt11) within the precardiac mesodermal cells [18][19] as well as within the cardiac tissues of the developing heart [20]. However, the relative contribution of either of these pathways depends on the organism or model system and the developmental stage under study.
In the case of canonical Wnt signaling, the canonical Drosophila Wnt gene wg functions as an inducer of cardiogenic gene programs [21] on one hand, while, on the other hand, the presence of Wnt inhibitors Dkk-1 and Crescent that disrupt Wnt/beta-catenin signaling trigger cardiac development in Xenopus embryos [22]. A similar down regulation of canonical Wnt signaling during cardiogenic induction was also observed in chick embryonic explants [23]. Alternatively, canonical Wnt signaling led to upregulated cardiac differentiation in embryonic stem cell lines [24]. Furthermore, a number of studies have demonstrated a biphasic effect of canonical Wnt signaling on cardiac development in Zebrafish embryos- during the pre-gastrula stage it promotes cardiac differentiation, whereas, during the gastrula stage it negatively regulates heart formation [25].
In vertebrates, some non-canonical Wnt ligands have also been identified as important molecular signals during cardiac development, with the most prominent of them being Wnt11. In this regard, several studies using quail, Xenopus, and mouse embryos have highlighted the functions of Wnt11 in promoting cardiac tissue formation, by inducing contractile activity within the tissue, and also in determining cardiomyocyte differentiation into ventricular lineages [26][18][27]. The involvement of another closely-related Wnt ligand, Wnt11R, has also been reported in Xenopus embryos, with signaling in both cases occurring in a JNK-dependent manner [28]. In agreement with such findings from vertebrate embryos, Wnt11 signaling is also known to guide both embryonic stem cell and adult somatic cell populations into cardiomyogenic lineages and therefore serves as a potential candidate that can drive the in vitro generation of cardiomyocytes for use in cardiac repair [29][25][30][31].
References
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, and Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc. Natl. Acad. Sci. U.S.A. 2005; 102(26):9182-7. [PMID: 15958533]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, and Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 2005; 24(4):742-52. [PMID: 15692570]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, and He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002; 108(6):837-47. [PMID: 11955436]
- Xing Y, Clements WK, Kimelman D, and Xu W. Crystal structure of a beta-catenin/axin complex suggests a mechanism for the beta-catenin destruction complex. Genes Dev. 2003; 17(22):2753-64. [PMID: 14600025]
- Kimelman D, and Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene 2006; 25(57):7482-91. [PMID: 17143292]
- Huber AH, Stewart DB, Laurents DV, Nelson WJ, and Weis WI. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J. Biol. Chem. 2000; 276(15):12301-9. [PMID: 11121423]
- Huber AH, and Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell 2001; 105(3):391-402. [PMID: 11348595]
- Bilic J, Huang Y, Davidson G, Zimmermann T, Cruciat C, Bienz M, and Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 2007; 316(5831):1619-22. [PMID: 17569865]
- Fagotto F, Glück U, and Gumbiner BM. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of beta-catenin. Curr. Biol. 1998; 8(4):181-90. [PMID: 9501980]
- Sharma M, Jamieson C, Johnson M, Molloy MP, and Henderson BR. Specific armadillo repeat sequences facilitate β-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358. J. Biol. Chem. 2011; 287(2):819-31. [PMID: 22110128]
- Xing Y, Takemaru K, Liu J, Berndt JD, Zheng JJ, Moon RT, and Xu W. Crystal structure of a full-length beta-catenin. Structure 2008; 16(3):478-87. [PMID: 18334222]
- Orsulic S, and Peifer M. An in vivo structure-function study of armadillo, the beta-catenin homologue, reveals both separate and overlapping regions of the protein required for cell adhesion and for wingless signaling. J. Cell Biol. 1996; 134(5):1283-300. [PMID: 8794868]
- Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, and Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996; 382(6592):638-42. [PMID: 8757136]
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, and Bejsovec A. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature 1998; 395(6702):604-8. [PMID: 9783586]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Züllig S, and Basler K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 2002; 109(1):47-60. [PMID: 11955446]
- Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, and Bienz M. A new nuclear component of the Wnt signalling pathway. Nat. Cell Biol. 2002; 4(5):367-73. [PMID: 11988739]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, and Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes Dev. 2004; 18(18):2225-30. [PMID: 15371335]
- Eisenberg CA, and Eisenberg LM. WNT11 promotes cardiac tissue formation of early mesoderm. Dev. Dyn. 1999; 216(1):45-58. [PMID: 10474165]
- Chapman SC, Brown R, Lees L, Schoenwolf GC, and Lumsden A. Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Dev. Dyn. 2004; 229(3):668-76. [PMID: 14991722]
- Eisenberg LM, and Eisenberg CA. Wnt signal transduction and the formation of the myocardium. Dev. Biol. 2006; 293(2):305-15. [PMID: 16563368]
- Park M, Wu X, Golden K, Axelrod JD, and Bodmer R. The wingless signaling pathway is directly involved in Drosophila heart development. Dev. Biol. 1996; 177(1):104-16. [PMID: 8660881]
- Schneider VA, and Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001; 15(3):304-15. [PMID: 11159911]
- Marvin MJ, Di Rocco G, Gardiner A, Bush SM, and Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001; 15(3):316-27. [PMID: 11159912]
- Nakamura T, Sano M, Songyang Z, and Schneider MD. A Wnt- and beta -catenin-dependent pathway for mammalian cardiac myogenesis. Proc. Natl. Acad. Sci. U.S.A. 2003; 100(10):5834-9. [PMID: 12719544]
- Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, and Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 2007; 104(23):9685-90. [PMID: 17522258]
- Flaherty MP, and Dawn B. Noncanonical Wnt11 signaling and cardiomyogenic differentiation. Trends Cardiovasc. Med. 2008; 18(7):260-8. [PMID: 19232955]
- Nagy II, Railo A, Rapila R, Hast T, Sormunen R, Tavi P, Räsänen J, and Vainio SJ. Wnt-11 signalling controls ventricular myocardium development by patterning N-cadherin and beta-catenin expression. Cardiovasc. Res. 2010; 85(1):100-9. [PMID: 19622544]
- Garriock RJ, D’Agostino SL, Pilcher KC, and Krieg PA. Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Dev. Biol. 2005; 279(1):179-92. [PMID: 15708567]
- Terami H, Hidaka K, Katsumata T, Iio A, and Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem. Biophys. Res. Commun. 2004; 325(3):968-75. [PMID: 15541384]
- Koyanagi M, Haendeler J, Badorff C, Brandes RP, Hoffmann J, Pandur P, Zeiher AM, Kühl M, and Dimmeler S. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J. Biol. Chem. 2005; 280(17):16838-42. [PMID: 15701629]
- Belema Bedada F, Technau A, Ebelt H, Schulze M, and Braun T. Activation of myogenic differentiation pathways in adult bone marrow-derived stem cells. Mol. Cell. Biol. 2005; 25(21):9509-19. [PMID: 16227601]


