What is tensin?
Tensin is a cytoskeleton scaffolding protein that was named for its ability to form a bridge that maintains tension between the actin filaments and cell-matrix adhesion sites (reviewed in [1]). A recent review of the molecular physiology of tensins, however, reveals that the evidence in support of actin binding is so weak that it is doubtful whether any tensin binds actin in vivo [2].
Tensin contains three actin-binding domains (ABDs) that allows it to form crosslinks along actin filaments; it also prevents actin assembly by capping actin filaments at the barbed end [3][4]. Tensin has numerous phosphorylation sites and multiple protein interaction domains for both structural components (e.g. paxillin, β-integrin [5]) and signaling molecules (e.g. Src, phosphatidylinositol 3-kinase [PI3K], focal adhesion kinase [FAK]) [6] (reviewed in [7]). Phosphorlyation of tensin corresponds with cell-ECM binding [8] and growth factor stimulation [9] (reviewed in [1]). Tensin forms a C-shaped structure [10] that binds focal adhesion components at both ends [11]. Tensin is also proposed to form a dimer via its carboxy-terminus and this association may be dependent upon its phosphorylation state [10].
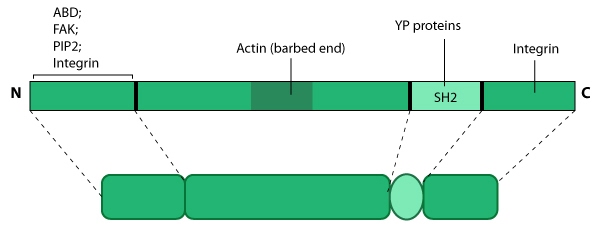
This schematic diagram illustrates the molecular organization of tensin and provides examples for how tensin is represented in figures throughout this resource. Relevant domains believed to be important for binding to actin and for protein-protein interactions are highlighted (reviewed in [7840759, 14592531]). PIP2- phosphatidylinositol [4, 5]-bis-phosphate; YP- phosphotyrosine; SH2- Src homology 2.
Tensin primarily localizes to sites of cell attachment such as focal adhesions [11] [12], elongated fibrillar structures (aka fibrillar adhesions)[13] and possibly other adhesive junctions [14]. Tensin serves as a link between signal transduction pathways and the actin cytoskeleton by forming a structural platform that regulates the assembly of focal adhesion components, phosphoproteins, and signaling molecules for processes such as cell migration [11][15] and tissue regeneration [16].
References
- Lo SH, Weisberg E, and Chen LB. Tensin: a potential link between the cytoskeleton and signal transduction. Bioessays 1994; 16(11):817-23. [PMID: 7840759]
- Haynie DT. Molecular physiology of the tensin brotherhood of integrin adaptor proteins. Proteins 2014; 82(7):1113-27. [PMID: 24634006]
- Lo SH, Janmey PA, Hartwig JH, and Chen LB. Interactions of tensin with actin and identification of its three distinct actin-binding domains. J. Cell Biol. 1994; 125(5):1067-75. [PMID: 8195290]
- Chuang JZ, Lin DC, and Lin S. Molecular cloning, expression, and mapping of the high affinity actin-capping domain of chicken cardiac tensin. J. Cell Biol. 1995; 128(6):1095-109. [PMID: 7896874]
- Calderwood DA, Fujioka Y, de Pereda JM, García-Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, and Ginsberg MH. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. U.S.A. 2003; 100(5):2272-7. [PMID: 12606711]
- Davis S, Lu ML, Lo SH, Lin S, Butler JA, Druker BJ, Roberts TM, An Q, and Chen LB. Presence of an SH2 domain in the actin-binding protein tensin. Science 1991; 252(5006):712-5. [PMID: 1708917]
- Lo SH. Tensin. Int. J. Biochem. Cell Biol. 2004; 36(1):31-4. [PMID: 14592531]
- Miyamoto S, Akiyama SK, and Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 1995; 267(5199):883-5. [PMID: 7846531]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, and Cavanagh HD. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest. Ophthalmol. Vis. Sci. 1999; 40(9):1959-67. [PMID: 10440249]
- Lo SH, An Q, Bao S, Wong WK, Liu Y, Janmey PA, Hartwig JH, and Chen LB. Molecular cloning of chick cardiac muscle tensin. Full-length cDNA sequence, expression, and characterization. J. Biol. Chem. 1994; 269(35):22310-9. [PMID: 8071358]
- Chen H, and Lo SH. Regulation of tensin-promoted cell migration by its focal adhesion binding and Src homology domain 2. Biochem. J. 2003; 370(Pt 3):1039-45. [PMID: 12495434]
- Wilkins JA, Risinger MA, and Lin S. Studies on proteins that co-purify with smooth muscle vinculin: identification of immunologically related species in focal adhesions of nonmuscle and Z-lines of muscle cells. J. Cell Biol. 1986; 103(4):1483-94. [PMID: 3095336]
- Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, and Kam Z. Molecular diversity of cell-matrix adhesions. J. Cell. Sci. 1999; 112 ( Pt 11):1655-69. [PMID: 10318759]
- Bockholt SM, Otey CA, Glenney JR, and Burridge K. Localization of a 215-kDa tyrosine-phosphorylated protein that cross-reacts with tensin antibodies. Exp. Cell Res. 1992; 203(1):39-46. [PMID: 1385191]
- Chen H, Duncan IC, Bozorgchami H, and Lo SH. Tensin1 and a previously undocumented family member, tensin2, positively regulate cell migration. Proc. Natl. Acad. Sci. U.S.A. 2002; 99(2):733-8. [PMID: 11792844]
- Ishii A, and Lo SH. A role of tensin in skeletal-muscle regeneration. Biochem. J. 2001; 356(Pt 3):737-45. [PMID: 11389681]


