What is Myosin Light Chain Kinase?
Myosin light chain kinase (MLCK) is a calcium/calmodulin-dependent serine/threonine kinase, belonging to the immunoglobulin superfamily. It phosphorylates the regulatory myosin light chains of myosin II, in order to facilitate myosin binding to actin and therefore aid contractility. Its role in contractile tissues is well-established, whilst comparatively less is known about its contractile function in non-muscle cells. The MLCK gene itself is alternatively spliced into several non-muscle and smooth muscle transcripts [1].
Functions of MLCK
MLCK is activated by calmodulin in response to an increase in intracellular calcium. It then goes on to phosphorylate regulatory myosin light chains at residues serine 19 and threonine 18 [2][3]. These phosphorylations enhance the ATPase activity of actin-activated myosin and so promotes myosin-driven contraction. It should however be noted that MLCK itself also has actin binding activity [4], via its N terminal domain [5][6]. Its ability to bind myofilaments specifically involves three repeats of the consensus sequence, DFRXXL, within its N terminal domain [7].
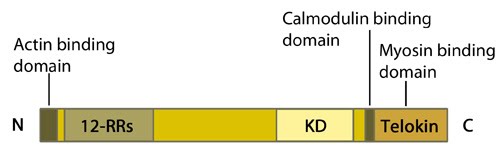
Schematic of the Myosin light chain kinase. 12-RRs = 12-residue long repeats; KD = kinase domain
In non-muscle cells MLCK activation of myosin II is implicated in a wide range of cellular processes, including cell spreading, migration and cytokinesis, as well as cell type specific processes such as neurite outgrowth and platelet morphogenesis (as reviewed in [8]). It should be noted that not all cells require a rise in intracellular calcium to induce motility or morphogenetic changes and in these cases the role of MLCK is unclear.
In cells that do require a change in intracellular calcium levels to promote cell motility, MLCK-dependent regulation is more evident. Generalities however cannot easily be drawn due to cell type specific variations in the role of MLCK. For example; decreased levels or functionality of MLCK is known to impair cell motility. However in eosinophils though motility is impaired lamellipodia formation persists [9], unlike in smooth muscle cells where both motility and lamellipodial formation are inhibited [10].
During mitosis, the cell undergoes significant changes in morphology requiring large scale reorganizations of the cytoskeleton. For example; during the formation of the cleavage furrow at the end of metaphase. Myosin II is known to be integral to this process as a component of the contractile ring and as such is tightly regulated through phosphorylation of its regulatory light chains (as reviewed in [11]). MLCK localizes to the spindle midzone during late metaphase and is maximally activated through calcium/calmodulin binding as the cleavage furrows begins to ingress. It is therefore well placed to regulate myosin II-dependent cytoskeletal rearrangements during ingression, though other kinases such as ROCK (Rho-associated protein kinase) and citron kinase are also likely to also contribute [12].
In addition to the major MLCK isoforms whose functions have been discussed above, the MLCK gene encodes telokin, also known as kinase-related protein (KRP). Telokin is generated using a promoter lying within an intronic region near the 3′ end of the MLCK gene. This generates a truncated gene product identical to the C terminus of MLCK. Telokin, of which there are two known isoforms, is expressed exclusively in smooth muscle and stabilizes unphosphorylated myosin [13].
References
- Lazar V, and Garcia JG. A single human myosin light chain kinase gene (MLCK; MYLK). Genomics 1999; 57(2):256-67. [PMID: 10198165]
- Ikebe M, and Hartshorne DJ. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J. Biol. Chem. 1985; 260(18):10027-31. [PMID: 3839510]
- Mabuchi Y, Mabuchi K, Stafford WF, and Grabarek Z. Modular structure of smooth muscle Myosin light chain kinase: hydrodynamic modeling and functional implications. Biochemistry 2010; 49(13):2903-17. [PMID: 20196616]
- Sellers JR, and Pato MD. The binding of smooth muscle myosin light chain kinase and phosphatases to actin and myosin. J. Biol. Chem. 1984; 259(12):7740-6. [PMID: 6330077]
- Kanoh S, Ito M, Niwa E, Kawano Y, and Hartshorne DJ. Actin-binding peptide from smooth muscle myosin light chain kinase. Biochemistry 1993; 32(34):8902-7. [PMID: 8364036]
- Lin PJ, Luby-Phelps K, and Stull JT. Binding of myosin light chain kinase to cellular actin-myosin filaments. J. Biol. Chem. 1997; 272(11):7412-20. [PMID: 9054442]
- Smith L, Su X, Lin P, Zhi G, and Stull JT. Identification of a novel actin binding motif in smooth muscle myosin light chain kinase. J. Biol. Chem. 1999; 274(41):29433-8. [PMID: 10506206]
- Kamm KE, and Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J. Biol. Chem. 2000; 276(7):4527-30. [PMID: 11096123]
- Walker JW, Gilbert SH, Drummond RM, Yamada M, Sreekumar R, Carraway RE, Ikebe M, and Fay FS. Signaling pathways underlying eosinophil cell motility revealed by using caged peptides. Proc. Natl. Acad. Sci. U.S.A. 1998; 95(4):1568-73. [PMID: 9465056]
- Kishi H, Mikawa T, Seto M, Sasaki Y, Kanayasu-Toyoda T, Yamaguchi T, Imamura M, Ito M, Karaki H, Bao J, Nakamura A, Ishikawa R, and Kohama K. Stable transfectants of smooth muscle cell line lacking the expression of myosin light chain kinase and their characterization with respect to the actomyosin system. J. Biol. Chem. 2000; 275(2):1414-20. [PMID: 10625693]
- Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005; 15(7):371-7. [PMID: 15935670]
- Chew T, Wolf WA, Gallagher PJ, Matsumura F, and Chisholm RL. A fluorescent resonant energy transfer-based biosensor reveals transient and regional myosin light chain kinase activation in lamella and cleavage furrows. J. Cell Biol. 2002; 156(3):543-53. [PMID: 11815633]
- Shirinsky VP, Vorotnikov AV, Birukov KG, Nanaev AK, Collinge M, Lukas TJ, Sellers JR, and Watterson DM. A kinase-related protein stabilizes unphosphorylated smooth muscle myosin minifilaments in the presence of ATP. J. Biol. Chem. 1993; 268(22):16578-83. [PMID: 8344938]


