What is integrin?
Introduction to integrin and its structure
Integrins are proteins that function mechanically, by attaching the cell cytoskeleton to the extracellular matrix (ECM), and biochemically, by sensing whether adhesion has occurred. The integrin family of proteins consists of alpha and beta subtypes, which form transmembrane heterodimers. Integrins function as adhesion receptors for extracellular ligands and transduce biochemical signals into the cell, through downstream effector proteins. Remarkably, they function bidirectionally, meaning they can transmit information both outside-in and inside-out (reviewed in [1][2]).
Integrin Structure
Each integrin heterodimer consists of an alpha (α) and a beta (β) subunit associated by noncovalent interactions forming an extracellular ligand-binding head, two multi-domain `legs’, two single-pass transmembrane helices and two short cytoplasmic tails. The α and β groups show no homology to each other,however, conserved regions are found among subtypes of both groups.
The α subunit leg consists of a thigh and 2 calf domains that support the ligand binding head formed by a β-propeller domain with 7 repeats forming the blades (shown as a cylinder in the figure below). Some of the propeller blade domains contain calcium binding EF-hand domains on the lower side; these allosterically affect ligand binding [3]. An additional αI (interactive) domain containing ~200 residues is present in some vertebrate α chains [4] (nine human α subtypes) between the propeller repeats 2 and 3 [5]. This contains a metal-ion dependent adhesion site (MIDAS) which is important for ligand binding.
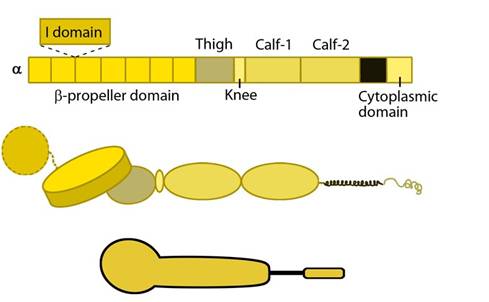
Integrin α subunit domains: Top: Linear domain arrangement. Middle: The globular structure formed by protein domains. Bottom: simplified version of the integrin α subunit. The αI domain is present in some subtypes of the α subunit.
The β subunit comprises of 4 cysteine-rich epidermal growth factor (EGF) repeats, a hybrid domain (split in sequence), an I-like domain (βI) and a plexin-sempahorin-integrin (PSI) domain. Similar to αI, the contains βI domain contains a MIDAS site for ligand binding and additional regulatory site “adjacent to MIDAS” or ADMIDAS, inhibited by Ca2+ and activated by Mn2+ [3] for ligand binding.
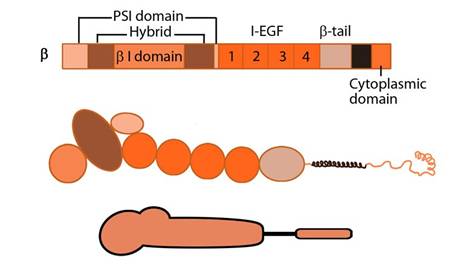
Integrin β subunit domains: Top: Linear domain arrangement. Middle: The globular structure formed by protein domains. Bottom: simplified version of the integrin β subunit.
i) Ligand binding
The βI domain binds ligand together with the β-propeller or with αI (if present) through MIDAS in a Mg2+ dependent fashion [6] at the interface in the headpiece. While Asp carboxyl group coordinates the βI MIDAS ion Mg2+, side chain hydrogen of the Arg of the RGD ligand binds directly to the Asp in domains 2 and 3 of β-propeller [7].
ii) Dimerization
Dimerization occurs via the β-propeller surface on the α chain and the hybrid domain in the β chain in the cytoplasm [8]. The sequences at these interacting surfaces seem to control the specificity of chain selection. The dimers have been shown to be stabilized and remain inactive by hydrophobic interactions and electrostatic salt bridges at the outer- and inner-membrane proximal regions respectively [9][10].
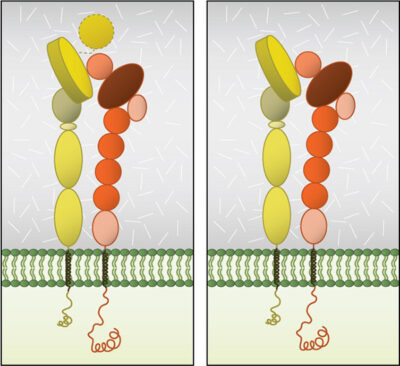
Globular domain structures of α and β subunits in a stable dimer. Ligand binding happens at the interface of the αI (left panel) or β-propeller (right panel) and the βI domain.
iii) Interactions
The cytoplasmic tail of β-chain is known to bind to protein adaptors through NPxY/F motifs [11]; this activates the integrins by breaking the salt bridge between the dimer (reviewed in [12][13]). In general, the adaptor proteins promote linkage to actin [14], however intermediate filaments have also been implicated via vimentin [15][16].
Protein adaptors that bind to integrin cytoplasmic tails:
- Structural adaptors (e.g. talin, tensin,) link integrins directly to the cytoskeleton
- Scaffolding adaptors (e.g. paxillin, kindlin) forms bridges between focal adhesion proteins
- Catalytic adaptors (e.g. focal adhesion kinase, integrin-linked kinase, Src) propagate signal transduction from adhesion sites. Interactions via α-tail are not well established due to sequence variability, however, α-tail is implicated in the cell-type specific integrin activation through binding proteins that modulate downstream signaling [17][18]. Phosphorylation state of cytoplasmic tail residues modulate the competition between adaptors for binding and hence the subsequent cytoskeletal interactions of integrins and response (reviewed in [19]).
The role of protein structure in ligand affinity modulation, signaling and dynamics of surface distribution of integrins is reviewed in [20].
Integrin functions
For liganded integrin to function as a bidirectional signal transmitter:
- Integrins undergo a process called activation, during which conformational changes expose the headpiece (βI and hybrid domain) for ligand binding [21][22][23][24]. This can be initiated by the binding of adaptor proteins and/or ligands.
- Adaptor proteins bind to the integrin cytoplasmic domains, thereby connecting integrin to the cytoskeleton.
- Integrins microcluster laterally for efficient ligand binding.
Upon activation, integrins are capable of triggering a variety of signal transduction cascades. The combination of α and β subtypes, for example, will affect different in vivo functions. As demonstrated by knockout mouse studies, and highlighted in the table below, these include cell behaviour and tissue organization (reviewed in [25][26][27][28]).
Which signalling pathway is initiated by integrin activation is based on the biological context, as well as the ligands bound (matrix components/growth factors). Depending on the combination of these factors, a variety of short-term and long-term responses may result [23]. Substrate stiffness has also been shown to affect the type of adhesion structure formed following integrin activation.
One study revealed the development of podosome-like adhesion structures in non-transformed fibroblasts grown on fluid, membrane based substrates. In this case, integrin was activated by membrane bound RGD (Arg-Gly-Asp) peptides. When grown on rigid surfaces, RGD-activated integrin would normally initiate the formation of focal adhesions [29]. The adhesion structures formed on the softer substrates had a similar morphology and makeup to classic podosomes found in macrophages. However, despite also being protrusive, the physiological function of these podosome-like structures remained unknown. The formation of these podosome-like structures in the absence of forces was mediated by p85beta recruitment and local PIP3 enrichment at the adhesion sites; both of which are not observed in focal adhesion formation. The increased production of PIP3 then caused N-WASP activation and RhoA -GAP ARAP3 recruitment, which downregulates RhoA-GTP level in podosome-forming cells.
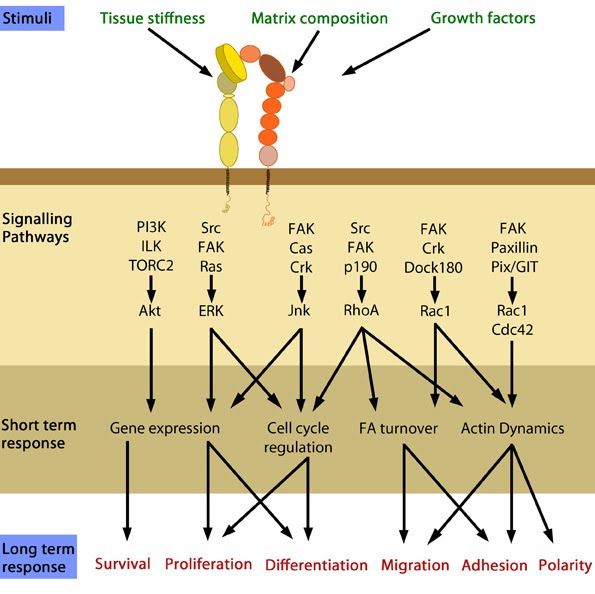
In response to physical/chemical properties of the matrix and growth factors in the environment (outside-in signaling), integrins bind ligands and get activated. Accordingly, a variety of signaling pathways can be triggered mainly through the different kinases as mentioned above. These can bring about changes in one or more cellular events (short term responses) that eventually result in global (long term) responses in cellular behavior.
References
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110(6):673-87. [PMID: 12297042]
- Harburger DS, and Calderwood DA. Integrin signalling at a glance. J. Cell. Sci. 2009; 122(Pt 2):159-63. [PMID: 19118207]
- Humphries MJ, Symonds EJH, and Mould AP. Mapping functional residues onto integrin crystal structures. Curr. Opin. Struct. Biol. 2003; 13(2):236-43. [PMID: 12727518]
- Johnson MS, Lu N, Denessiouk K, Heino J, and Gullberg D. Integrins during evolution: evolutionary trees and model organisms. Biochim. Biophys. Acta 2009; 1788(4):779-89. [PMID: 19161977]
- Larson RS, Corbi AL, Berman L, and Springer T. Primary structure of the leukocyte function-associated molecule-1 alpha subunit: an integrin with an embedded domain defining a protein superfamily. J. Cell Biol. 1989; 108(2):703-12. [PMID: 2537322]
- Lee JO, Bankston LA, Arnaout MA, and Liddington RC. Two conformations of the integrin A-domain (I-domain): a pathway for activation? Structure 1995; 3(12):1333-40. [PMID: 8747460]
- Xiao T, Takagi J, Coller BS, Wang J, and Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature 2004; 432(7013):59-67. [PMID: 15378069]
- Humphries MJ. Integrin structure. Biochem. Soc. Trans. 2000; 28(4):311-39. [PMID: 10961914]
- Hughes PE, Diaz-Gonzalez F, Leong L, Wu C, McDonald JA, Shattil SJ, and Ginsberg MH. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J. Biol. Chem. 1996; 271(12):6571-4. [PMID: 8636068]
- Lau T, Kim C, Ginsberg MH, and Ulmer TS. The structure of the integrin alphaIIbbeta3 transmembrane complex explains integrin transmembrane signalling. EMBO J. 2009; 28(9):1351-61. [PMID: 19279667]
- Calderwood DA, Fujioka Y, de Pereda JM, García-Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, and Ginsberg MH. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. U.S.A. 2003; 100(5):2272-7. [PMID: 12606711]
- Barczyk M, Carracedo S, and Gullberg D. Integrins. Cell Tissue Res. 2009; 339(1):269-80. [PMID: 19693543]
- Legate KR, and Fässler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J. Cell. Sci. 2009; 122(Pt 2):187-98. [PMID: 19118211]
- Geiger B, Spatz JP, and Bershadsky AD. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009; 10(1):21-33. [PMID: 19197329]
- Nievers MG, Schaapveld RQ, and Sonnenberg A. Biology and function of hemidesmosomes. Matrix Biol. 1999; 18(1):5-17. [PMID: 10367727]
- Bhattacharya R, Gonzalez AM, Debiase PJ, Trejo HE, Goldman RD, Flitney FW, and Jones JCR. Recruitment of vimentin to the cell surface by beta3 integrin and plectin mediates adhesion strength. J. Cell. Sci. 2009; 122(Pt 9):1390-400. [PMID: 19366731]
- Tohyama Y, Katagiri K, Pardi R, Lu C, Springer TA, and Kinashi T. The critical cytoplasmic regions of the alphaL/beta2 integrin in Rap1-induced adhesion and migration. Mol. Biol. Cell 2003; 14(6):2570-82. [PMID: 12808052]
- Yuan W, Leisner TM, McFadden AW, Wang Z, Larson MK, Clark S, Boudignon-Proudhon C, Lam SC, and Parise LV. CIB1 is an endogenous inhibitor of agonist-induced integrin alphaIIbbeta3 activation. J. Cell Biol. 2006; 172(2):169-75. [PMID: 16418530]
- Gahmberg CG, Fagerholm SC, Nurmi SM, Chavakis T, Marchesan S, and Grönholm M. Regulation of integrin activity and signalling. Biochim. Biophys. Acta 2009; 1790(6):431-44. [PMID: 19289150]
- Luo B, Carman CV, and Springer TA. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007; 25:619-47. [PMID: 17201681]
- Calderwood DA. Integrin activation. J. Cell. Sci. 2004; 117(Pt 5):657-66. [PMID: 14754902]
- Puklin-Faucher E, Gao M, Schulten K, and Vogel V. How the headpiece hinge angle is opened: New insights into the dynamics of integrin activation. J. Cell Biol. 2006; 175(2):349-60. [PMID: 17060501]
- Askari JA, Tynan CJ, Webb SED, Martin-Fernandez ML, Ballestrem C, and Humphries MJ. Focal adhesions are sites of integrin extension. J. Cell Biol. 2010; 188(6):891-903. [PMID: 20231384]
- Legate KR, Wickström SA, and Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009; 23(4):397-418. [PMID: 19240129]
- Sheppard D. In vivo functions of integrins: lessons from null mutations in mice. Matrix Biol. 2000; 19(3):203-9. [PMID: 10936445]
- Taverna D, Moher H, Crowley D, Borsig L, Varki A, and Hynes RO. Increased primary tumor growth in mice null for beta3- or beta3/beta5-integrins or selectins. Proc. Natl. Acad. Sci. U.S.A. 2004; 101(3):763-8. [PMID: 14718670]
- Taverna D, Crowley D, Connolly M, Bronson RT, and Hynes RO. A direct test of potential roles for beta3 and beta5 integrins in growth and metastasis of murine mammary carcinomas. Cancer Res. 2005; 65(22):10324-9. [PMID: 16288021]
- Reynolds LE, Conti FJ, Lucas M, Grose R, Robinson S, Stone M, Saunders G, Dickson C, Hynes RO, Lacy-Hulbert A, and Hodivala-Dilke K. Accelerated re-epithelialization in beta3-integrin-deficient- mice is associated with enhanced TGF-beta1 signaling. Nat. Med. 2005; 11(2):167-74. [PMID: 15654327]
- Yu C, Rafiq NBM, Krishnasamy A, Hartman KL, Jones GE, Bershadsky AD, and Sheetz MP. Integrin-matrix clusters form podosome-like adhesions in the absence of traction forces. Cell Rep 2013; 5(5):1456-68. [PMID: 24290759]


