What is clathrin-mediated endocytosis?
Clathrin-mediated endocytosis (CME) is a vesicular transport event that facilitates the internalization and recycling of receptors engaged in a variety of processes, including signal transduction (G-protein and tyrosine kinase receptors), nutrient uptake and synaptic vesicle reformation [1]. Two classical examples of CME are iron-bound transferrin recycling and the uptake of low-density lipoprotein (LDL).
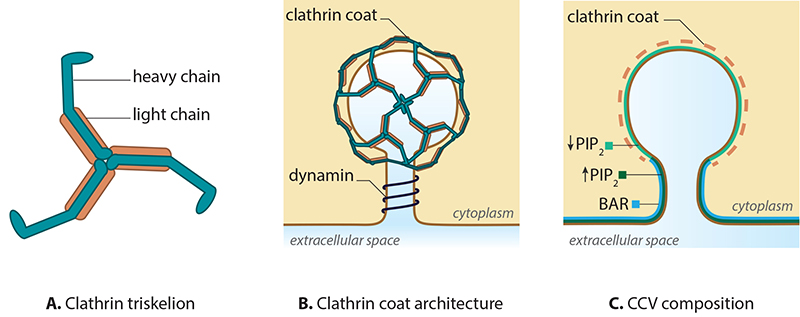
A. Clathrin is a triskelion shaped scaffold protein. B. Clathrin assembles to form a coat around a mature vesicle. C. Formation of the clathrin coated vesicle requires changes in membrane curvature, which is driven by PIP2 levels and BAR protein binding.
CME is characterized by the involvement of clathrin, which is a triskelion-shaped scaffold protein composed of three heavy and three light chains [2][3][4]. Clathrins polymerize around the cytoplasmic face of the invaginated membrane and act as a reinforced mould in which the membrane vesicle may form without direct association with the membrane [5]. Dissociation of the coat occurs rapidly following scission of the vesicle from the membrane.
Initiation of the clathrin complex formation requires the accumulation of phosphatidylinositol‑4,5‑bisphosphate (PIP2) and adaptor proteins, such as AP-2, at the pinching site [6][7][8]. In the case of clathrin-coated vesicles (CCV) formed at the trans-Golgi apparatus (TGA), AP-1 is essential [9][10].
Growth of the clathrin coated pit requires BAR (Bin/Amphiphysin/Rvs) domain proteins [11][12][13] and reorganization of the actin network [14]. The final scission step involves BAR domain proteins, dynamin and the dephosphorylation of PIP2. The latter step is suggested to function within a positive feedback loop, with regards to phosphatase activity [15][16][17]. The vesicles are then transported and sorted, based on receptor type or membrane composition [18], to the various destinations including the trans-Golgi network, endosomes and vacuoles.
References
- Takei K, and Haucke V. Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell Biol. 2001; 11(9):385-91. [PMID: 11514193]
- Kirchhausen T. Imaging endocytic clathrin structures in living cells. Trends Cell Biol. 2009; 19(11):596-605. [PMID: 19836955]
- ter Haar E, Musacchio A, Harrison SC, and Kirchhausen T. Atomic structure of clathrin: a beta propeller terminal domain joins an alpha zigzag linker. Cell 1998; 95(4):563-73. [PMID: 9827808]
- Edeling MA, Smith C, and Owen D. Life of a clathrin coat: insights from clathrin and AP structures. Nat. Rev. Mol. Cell Biol. 2006; 7(1):32-44. [PMID: 16493411]
- McMahon HT, and Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011; 12(8):517-33. [PMID: 21779028]
- Mousavi SA, Malerød L, Berg T, and Kjeken R. Clathrin-dependent endocytosis. Biochem. J. 2004; 377(Pt 1):1-16. [PMID: 14505490]
- Haucke V. Phosphoinositide regulation of clathrin-mediated endocytosis. Biochem. Soc. Trans. 2005; 33(Pt 6):1285-9. [PMID: 16246100]
- Jost M, Simpson F, Kavran JM, Lemmon MA, and Schmid SL. Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated vesicle formation. Curr. Biol. 8(25):1399-402. [PMID: 9889104]
- Radhakrishnan K, Baltes J, Creemers JWM, and Schu P. Trans-Golgi network morphology and sorting is regulated by prolyl-oligopeptidase-like protein PREPL and the AP-1 complex subunit μ1A. J. Cell. Sci. 2013; 126(Pt 5):1155-63. [PMID: 23321636]
- Ahle S, Mann A, Eichelsbacher U, and Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988; 7(4):919-29. [PMID: 3402440]
- Dawson JC, Legg JA, and Machesky LM. Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol. 2006; 16(10):493-8. [PMID: 16949824]
- Ferguson SM, Ferguson S, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, Destaing O, Ko G, Takasaki J, Cremona O, O’ Toole E, and De Camilli P. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev. Cell 2009; 17(6):811-22. [PMID: 20059951]
- Sundborger A, Soderblom C, Vorontsova O, Evergren E, Hinshaw JE, and Shupliakov O. An endophilin-dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling. J. Cell. Sci. 2011; 124(Pt 1):133-43. [PMID: 21172823]
- Schafer DA. Coupling actin dynamics and membrane dynamics during endocytosis. Curr. Opin. Cell Biol. 2002; 14(1):76-81. [PMID: 11792548]
- Neumann S, and Schmid SL. Dual role of BAR domain-containing proteins in regulating vesicle release catalyzed by the GTPase, dynamin-2. J. Biol. Chem. 2013; 288(35):25119-28. [PMID: 23861397]
- Meinecke M, Boucrot E, Camdere G, Hon W, Mittal R, and McMahon HT. Cooperative recruitment of dynamin and BIN/amphiphysin/Rvs (BAR) domain-containing proteins leads to GTP-dependent membrane scission. J. Biol. Chem. 2013; 288(9):6651-61. [PMID: 23297414]
- Cremona O, Di Paolo G, Wenk MR, Lüthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, McCormick DA, and De Camilli P. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 1999; 99(2):179-88. [PMID: 10535736]
- Madshus IH, and Stang E. Internalization and intracellular sorting of the EGF receptor: a model for understanding the mechanisms of receptor trafficking. J. Cell. Sci. 2009; 122(Pt 19):3433-9. [PMID: 19759283]


