What is axon guidance and the growth cone?
Axon guidance is an important step in neural development. It allows growing axons to reach specific destinations and ultimately form the complex neuronal networks throughout the body. Although many aspects of this mechanism remains unclear, it is well established that a dynamic and highly motile actin-based structure found at the growing end of a developing axon, known as a growth cone, facilitates this process.
The Growth Cone
Growth cones facilitate axon growth and guidance by bundling and extending actin filaments into structures known as filopodia and microspikes. Binding of filopodia and adhesion receptors to particular extracellular matrix (ECM) components or ligands is translated into actin filament assembly, cytoskeleton remodeling and force-driven motility. These events culminate in the growth of the neuron towards its target.
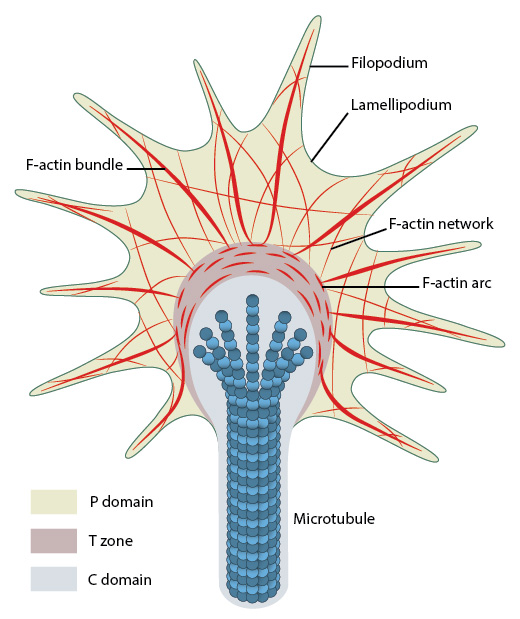
Organization of cytoskeletal components (actin filaments and microtubules) in the growth cone.
Growth cones contain a number of cytoskeletal components that are organized into three regions; the peripheral (P) domain, the transitional (T) domain and the central (C) domain [1].
- The P domain is primarily composed of unipolar actin filament bundles embedded in a less polar actin network. It contains dynamic lamellipodia and filopodia. Microtubules are also transiently found within this domain.
- The T domain is a thin interface between the P and C domains.
- The C domain is located in the center of the growth cone nearest the axon. It is primarily composed of microtubules and contains numerous organelles and vesicles.
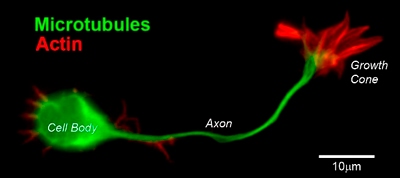
CD1 mouse spinal commissural neuron with growth cone: Image provided by Simon Moore, Research Fellow, Columbia University
A number of cytoskeletal-associated proteins are present in growth cones that anchor actin filaments and microtubules to each other, such as myosin II [2], or to the membrane, for example, talin [3], and to other cytoskeletal components. Molecular motors present in growth cones produce the forces needed for growth cone migration (e.g. myosin II [4]) and vesicle transport in and out of the growth cone (e.g. kinesin superfamily protein member 4, KIF4 [5]).
What causes growth cone collapse?
Growth cone collapse is a complex phenomenon involving numerous signal pathways including Rho-GTPases [6], ADF [7], and various kinases [8][9]. A model for filopodia collapse in growth cones was created using the guidance signal, semaphorin IIIA (SemaIIIA; collapsin-1). SemaIIIA causes termination of protrusive activity and growth cone collapse [10] through decreased phosphorylation of the ezrin–radixin–moesin (ERM) family of F-actin binding proteins [11]. Phosphorylation of ERM proteins activates the F-actin binding domain and regulates filopodia assembly/protrusion by linking filopodial membranes with F-actin (reviewed in [12]). Inactivation of the phosphoinositide 3-kinase (PI3K) signal pathway by SemaIIIA may also be linked to reduced ERM protein activity and growth cone collapse [11].
References
- Suter DM, and Forscher P. An emerging link between cytoskeletal dynamics and cell adhesion molecules in growth cone guidance. Curr. Opin. Neurobiol. 1998; 8(1):106-16. [PMID: 9568398]
- Burnette DT, Ji L, Schaefer AW, Medeiros NA, Danuser G, and Forscher P. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev. Cell 2008; 15(1):163-9. [PMID: 18606149]
- Sydor AM, Su AL, Wang FS, Xu A, and Jay DG. Talin and vinculin play distinct roles in filopodial motility in the neuronal growth cone. J. Cell Biol. 1996; 134(5):1197-207. [PMID: 8794861]
- Peretti D, Peris L, Rosso S, Quiroga S, and Cáceres A. Evidence for the involvement of KIF4 in the anterograde transport of L1-containing vesicles. J. Cell Biol. 2000; 149(1):141-52. [PMID: 10747093]
- Medeiros NA, Burnette DT, and Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 2006; 8(3):215-26. [PMID: 16501565]
- Liu BP, and Strittmatter SM. Semaphorin-mediated axonal guidance via Rho-related G proteins. Curr. Opin. Cell Biol. 2001; 13(5):619-26. [PMID: 11544032]
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, and Yahara I. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat. Neurosci. 2001; 4(4):367-73. [PMID: 11276226]
- Sasaki Y, Cheng C, Uchida Y, Nakajima O, Ohshima T, Yagi T, Taniguchi M, Nakayama T, Kishida R, Kudo Y, Ohno S, Nakamura F, and Goshima Y. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 2002; 35(5):907-20. [PMID: 12372285]
- Eickholt BJ, Walsh FS, and Doherty P. An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. J. Cell Biol. 2002; 157(2):211-7. [PMID: 11956225]
- Luo Y, Raible D, and Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 1993; 75(2):217-27. [PMID: 8402908]
- Gallo G. Semaphorin 3A inhibits ERM protein phosphorylation in growth cone filopodia through inactivation of PI3K. Dev Neurobiol 2008; 68(7):926-33. [PMID: 18327764]
- Bretscher A, Edwards K, and Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002; 3(8):586-99. [PMID: 12154370]


