What is autophagy?
Autophagy, meaning self-eating, is an intracellular degradation system wherein unwanted cargo, such as old or damaged organelles, unneeded proteins, as well as pathogenic agents, are digested and the macromolecular contents from the digestion are released back into the cytosol [1]. First described in 1963 by Christian de Duve [2], autophagy involves the sequestration of cell organelles and cytoplasmic material into double-membrane vesicles called autophagosomes and their subsequent delivery to the lysosomes for degradation by the lysosomal hydrolases [1][3][4].
Mechanism of Autophagy
Autophagy in eukaryotes comprises of three different pathways, namely macroautophagy, microautophagy and chaperone-mediated autophagy. Although all of the three mechanistically different pathways culminate in the lysosomal degradation of cellular cargo, macroautophagy is the most extensively studied and is discussed here briefly [3].
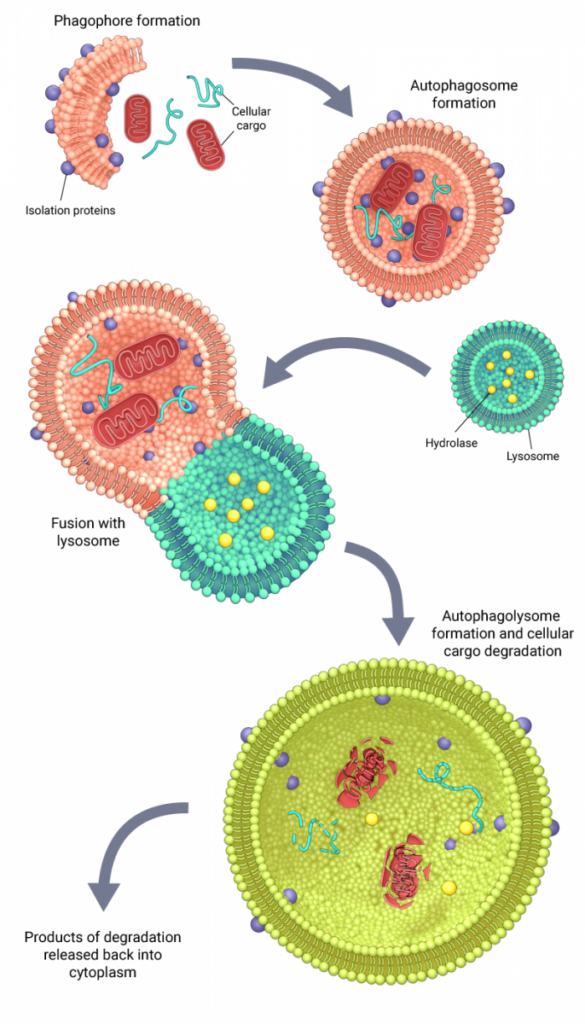
Schematic depicting the mechanism of autophagy
The mechanism of macroautophagy is conserved among eukaryotes and is characterized by the encapsulation of cellular cargo into double-membrane vesicles called autophagosomes. In yeast, the formation of autophagosomes around the targeted cargo is mediated by autophagy-related (Atg) proteins that are recruited hierarchically to the phagophore assembly site or the preautophagosomal structure (PAS). At the PAS, initiator protein complexes facilitate the de novo synthesis of a double membrane structure called a phagophore or an isolation membrane, the lipid components for which are derived from the golgi-endosome system [4][5]. In mammals, where a distinct PAS-like structure has not been identified, multiple cellular organelles, including the plasma membrane, are known to serve as origins for the assembly of a phagophore [6]. Upon the recruitment of other Atg proteins, the isolation membrane gets extended into a phagophore, which eventually fuses at its free ends to form an autophagosome, which now surrounds and sequesters the cargo. Once formed, autophagosomes undergo a maturation process as they are transported along the endocytic pathway, before fusing with the lysosomes to form autophagolysosomes. The cellular cargo delivered by the autophagosomes are then degraded by the hydrolytic enzymes of the lysosomes and the products of degradation are released back into the cytoplasm for cell use [6][4].
The other two autophagy pathways do not require the formation of an autophagosome. In microautophagy, the lysosome directly engulfs portions of the cytoplasm [7], whereas in chaperone-mediated autophagy, specific chaperone proteins bind to the cargo and transport it across the lysosomal membrane for degradation [8].
Physiological relevance of Autophagy
Autophagy is a both a stress-management system and a means of homeostatic control in cells, and is therefore regulated differently under varying cellular conditions. For instance, in cells functioning normally under stress-free conditions, a basal level of autophagy ensures that old, damaged organelles and proteins are rapidly digested and that the contents from digestion are recycled back into the cytosol, so that the availability of cellular components are regulated for various cellular functions. But, in response to various types of cellular stresses such as nutrient starvation, oxidative stress, radiation or anticancer therapy, the autophagic machinery is upregulated in order to rapidly detoxify cells as well as to increase the recycling of cellular components to keep up with intensified cell function [9][10][11][12][13]. Furthermore, in normal physiology as well as under pathological conditions, autophagy is known to play a direct role in inhibiting apoptosis by regulating interactions between autophagy protein Beclin-1 and apoptosis regulator Bcl-2 [14][15]. However, in the absence of stringent spatio-temporal regulation, excessive autophagy can function as an alternative cell-death pathway [16]. Therefore, dysregulated autophagy has been associated with the onset and progression of diseases such as cancer, neurodegenerative and autoimmune disorders, and many more [17].
Mechanical stress and Autophagy
When autophagy is acting as a pro-survival mechanism primarily induced by stress, it can be naturally regulated by mechanical stresses such as compression, stretching or shear stress due to fluid flow. Consistent with this, a number of studies have highlighted how cells respond to mechanical stresses by regulating autophagy levels and how this could have implications in both physiological as well as pathophysiological conditions. For instance, in response to a mechanical stimulus such as exercise, the mineralization capacity of mechano-sensitive osteoblasts is stimulated, leading to enhanced bone formation and remodeling. In relation to this, recent studies on UMR-106 rat osteoblast cell line have shown an increase in autophagy during mineralization and suggested a link between low bone density and deficiency of the autophagy protein Atg5 [18]. Such studies are indicative of the role of autophagy in regulating bone remodeling in response to mechanical stimuli.
Another recent study has demonstrated that cells induce autophagy in response to compressive stresses. Following application of compressive forces of up to 1kPa, which is within the range of normal physiological forces experienced by cells, there was a transient increase in the rate of autophagosome formation [19]. This transient increase was suggested to function as a cellular stress management system until the cell is able to adapt to physical changes in their environment. On the other hand, excessive mechanical stresses can have an opposite effect, leading to suppression of autophagy. In a recent study by Carames et al, human and mouse cartilage explants subjected to high impact mechanical injuries underwent cell death, which was associated with a significant decrease in expression of autophagy markers. Interestingly, pharmacological stimulation of autophagy by rapamycin protected against cell death, highlighting the interaction between autophagy and mechanical stress in maintaining healthy cells [20].
References
- Benbrook DM, and Long A. Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp. Oncol. 2012; 34(3):286-97. [PMID: 23070014]
- DE DUVE C. The lysosome. Sci. Am. 1963; 208:64-72. [PMID: 14025755]
- Yang Z, and Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2009; 22(2):124-31. [PMID: 20034776]
- Polson HEJ, de Lartigue J, Rigden DJ, Reedijk M, Urbé S, Clague MJ, and Tooze SA. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy 2010; 6(4):506-22. [PMID: 20505359]
- Ohashi Y, and Munro S. Membrane delivery to the yeast autophagosome from the Golgi-endosomal system. Mol. Biol. Cell 2010; 21(22):3998-4008. [PMID: 20861302]
- Abounit K, Scarabelli TM, and McCauley RB. Autophagy in mammalian cells. World J Biol Chem 2012; 3(1):1-6. [PMID: 22312452]
- Li W, Li J, and Bao J. Microautophagy: lesser-known self-eating. Cell. Mol. Life Sci. 2011; 69(7):1125-36. [PMID: 22080117]
- Cuervo AM, and Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2013; 24(1):92-104. [PMID: 24281265]
- Onodera J, and Ohsumi Y. Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. J. Biol. Chem. 2005; 280(36):31582-6. [PMID: 16027116]
- Kurihara Y, Kanki T, Aoki Y, Hirota Y, Saigusa T, Uchiumi T, and Kang D. Mitophagy plays an essential role in reducing mitochondrial production of reactive oxygen species and mutation of mitochondrial DNA by maintaining mitochondrial quantity and quality in yeast. J. Biol. Chem. 2011; 287(5):3265-72. [PMID: 22157017]
- Lee J, Giordano S, and Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 2012; 441(2):523-40. [PMID: 22187934]
- Fouillet A, Levet C, Virgone A, Robin M, Dourlen P, Rieusset J, Belaidi E, Ovize M, Touret M, Nataf S, and Mollereau B. ER stress inhibits neuronal death by promoting autophagy. Autophagy 2012; 8(6):915-26. [PMID: 22660271]
- Yorimitsu T, Nair U, Yang Z, and Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem. 2006; 281(40):30299-304. [PMID: 16901900]
- Decuypere J, Parys JB, and Bultynck G. Regulation of the autophagic bcl-2/beclin 1 interaction. Cells 2012; 1(3):284-312. [PMID: 24710477]
- Wang Z, Shi X, Yin J, Zuo G, Zhang J, and Chen G. Role of autophagy in early brain injury after experimental subarachnoid hemorrhage. J. Mol. Neurosci. 2011; 46(1):192-202. [PMID: 21728063]
- Maiuri MC, Zalckvar E, Kimchi A, and Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007; 8(9):741-52. [PMID: 17717517]
- Jiang P, and Mizushima N. Autophagy and human diseases. Cell Res. 2013; 24(1):69-79. [PMID: 24323045]
- Nollet M, Santucci-Darmanin S, Breuil V, Al-Sahlanee R, Cros C, Topi M, Momier D, Samson M, Pagnotta S, Cailleteau L, Battaglia S, Farlay D, Dacquin R, Barois N, Jurdic P, Boivin G, Heymann D, Lafont F, Lu SS, Dempster DW, Carle GF, and Pierrefite-Carle V. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy 2014; 10(11):1965-77. [PMID: 25484092]
- King JS, Veltman DM, and Insall RH. The induction of autophagy by mechanical stress. Autophagy 2011; 7(12):1490-9. [PMID: 22024750]
- Caramés B, Taniguchi N, Seino D, Blanco FJ, D’Lima D, and Lotz M. Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 2011; 64(4):1182-92. [PMID: 22034068]


