What are septins?
Septins are highly conserved small GTP binding proteins of 30-65 kDa, recognized as a unique component of the cytoskeleton {reviewed in [1]}. Septin monomers assemble into hetero oligomeric complexes and polymerize into filaments which can associate with the membrane and other cytoskeletal filaments like actin and microtubules. Septins additionally form other higher order structures like rings and gauzes. Unlike actin filaments and microtubules, septin filaments are nonpolar and can polymerize on both ends, similar to intermediate filaments.
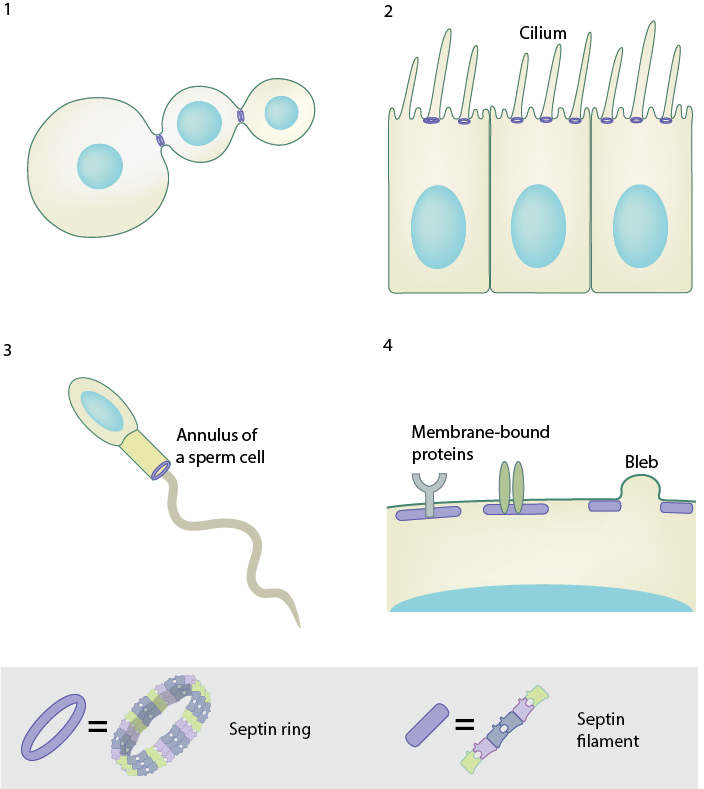
During cytokinesis in budding yeast, septin rings assemble at the bud neck, serving as a scaffold for the recruitment of proteins and cytoskeletal elements required for cytokinesis. Septins are in fact required for the assembly of the contractile actomyosin ring that drives cytokinesis. 2. Septin ring separates the ciliary membrane from the plasma membrane, functioning as a diffusion barrier and supporting the formation of the cilium. 3. Septin ring at the annulus of the mammalian spermatozoon forms a diffusion barrier between the anterior and posterior of the tail. 4. Septins at the plasma membrane acts as a scaffold for membrane-bound proteins and provides rigidity to the cell. Septin-mediated cell rigidity is important for regulating cell shape and directional motility. They are also required to retract membranes during blebbing and in vesicle fusion.
Septins were first identified in the budding yeast Saccharomyces cerevisiae and were named as they were found to localize to the septating bud neck in dividing cells. Septins are essential for cytokinesis in budding yeast [2]. During cytokinesis in budding yeast, septin rings assemble at the bud neck, serving as a scaffold for the recruitment of proteins and cytoskeletal elements required for cytokinesis. Septins are in fact required for the assembly of the contractile actomyosin ring that drives cytokinesis [3]. Further research on mammalian septins revealed that they are required for cytokinesis [4], spermiogenesis [5], neurogenesis [6] [7] and ciliogenesis [5] in metazoans {reviewed in [8]}. Septins also play a role in tumorigenesis [9] [10], neurological disorders, [11] infections and host defense mechanisms [12] [13] [14] and {reviewed in [15]}.
The multiple roles of septins outlined above can be attributed to the various functions of septins at a molecular level. Mainly, septin subunits interact with the phosphoinositides in the membrane in order to form membrane subdomains or compartments, thereby functioning as diffusion barriers and additionally playing a role in membrane trafficking [16] {reviewed in [17]}. The sites where septins associate possess some degree of curvature and consistent with this septins can detect membrane curvature at a micron scale, for example at the cytokinetic furrow [18] [19]. Septins can also serve as scaffolds for the site-specific recruitment of proteins and other cytoskeletal elements like actin and microtubules [20].
Septin homologs and structure
The number of septin encoding genes differs widely in different eukaryotes from 2 in nematodes to 13 in humans. The 13 septin genes in humans are, SEPT1-SEPT12 and SEPT14, and are classified based on sequence similarity into four homology groups: SEPT2, SEPT3, SEPT6 and SEPT7. Septins belong to the superclass of phosphate-binding loop (P-loop) NTPases which includes the oncogenic RAS GTPases. Structurally, the septins have an amino terminal region with Proline-rich domains, a carboxy terminal with coiled-coil domains and a central core region with a polybasic region that can bind to negatively charged phosphoinositides on the plasma membrane, a GTP-binding domain and 53 highly conserved amino acids of unknown function called the septin unique element (SUE).
Septin subunits interact through their GTP-binding domain (G interface) and N-terminal and C-terminal regions (NC interface) that come close together upon folding. The septin hetero complex is formed when septin monomers from different homology groups interact with one another through the G and NC interface in a palindromic order. In humans, septins initially assemble as complexes that contain either three or four septins, with each present in two copies When these septins join end-to-end, a non-polar heterohexamer (with three septins on either end) or heterooctamer complex (with four septins on either end) is formed. These complexes can then join laterally to form filaments, bundles or higher-order structures like a ring or cage {reviewed in [1]}.
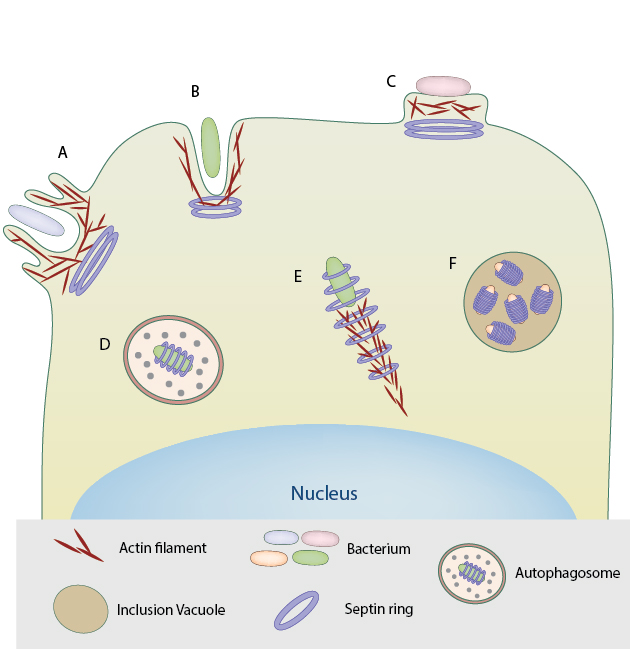
Septin rings assemble at the base of macropinosome-like structures induced by triggering bacteria for entry into host cell. B. Septin assembly at the site of zipper mode of entry into host cell by bacterial pathogens such as S. flexneri and L.monocytogenes. C . Septin rings at the site of actin pedestals induced by extracellular pathogens like EPEC. D. In the case of S.flexneri, the septin rings form cage-like structures around the bacteria that are recognized by the host immune system and targeted for autophagy. E. Septin rings form around actin comet tails of bacteria such as S.flexneri, restricting their motility and proliferation. F. Septins are required for the formation and extrusion of inclusion vacuoles by Chlamydia trachomatis essential for their replication and propagation within the host.
References
- Mostowy S, and Cossart P. Septins: the fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2012; 13(3):183-94. [PMID: 22314400]
- Glomb O, and Gronemeyer T. Septin Organization and Functions in Budding Yeast. Front Cell Dev Biol 2016; 4:123. [PMID: 27857941]
- Meitinger F, and Palani S. Actomyosin ring driven cytokinesis in budding yeast. Semin. Cell Dev. Biol. 2016; 53:19-27. [PMID: 26845196]
- Menon MB, Sawada A, Chaturvedi A, Mishra P, Schuster-Gossler K, Galla M, Schambach A, Gossler A, Förster R, Heuser M, Kotlyarov A, Kinoshita M, and Gaestel M. Genetic deletion of SEPT7 reveals a cell type-specific role of septins in microtubule destabilization for the completion of cytokinesis. PLoS Genet. 2014; 10(8):e1004558. [PMID: 25122120]
- Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, Goto M, Okubo K, Nishiyama H, Ogawa O, Takahashi C, Itohara S, Nishimune Y, Noda M, and Kinoshita M. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev. Cell 2005; 8(3):343-52. [PMID: 15737930]
- Tsang CW, Fedchyshyn M, Harrison J, Xie H, Xue J, Robinson PJ, Wang L, and Trimble WS. Superfluous role of mammalian septins 3 and 5 in neuronal development and synaptic transmission. Mol. Cell. Biol. 2008; 28(23):7012-29. [PMID: 18809578]
- Ageta-Ishihara N, Miyata T, Ohshima C, Watanabe M, Sato Y, Hamamura Y, Higashiyama T, Mazitschek R, Bito H, and Kinoshita M. Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation. Nat Commun 2013; 4:2532. [PMID: 24113571]
- Saarikangas J, and Barral Y. The emerging functions of septins in metazoans. EMBO Rep. 2011; 12(11):1118-26. [PMID: 21997296]
- Angelis D, and Spiliotis ET. Septin Mutations in Human Cancers. Front Cell Dev Biol 2016; 4:122. [PMID: 27882315]
- Poüs C, Klipfel L, and Baillet A. Cancer-Related Functions and Subcellular Localizations of Septins. Front Cell Dev Biol 2016; 4:126. [PMID: 27878118]
- Takehashi M, Alioto T, Stedeford T, Persad AS, Banasik M, Masliah E, Tanaka S, and Ueda K. Septin 3 gene polymorphism in Alzheimer’s disease. Gene Expr. 2004; 11(5-6):263-70. [PMID: 15200238]
- Torraca V, and Mostowy S. Septins and Bacterial Infection. Front Cell Dev Biol 2016; 4:127. [PMID: 27891501]
- Bridges AA, and Gladfelter AS. Fungal pathogens are platforms for discovering novel and conserved septin properties. Curr. Opin. Microbiol. 2014; 20:42-8. [PMID: 24879478]
- Mostowy S. Multiple roles of the cytoskeleton in bacterial autophagy. PLoS Pathog. 2014; 10(11):e1004409. [PMID: 25412422]
- Peterson EA, and Petty EM. Conquering the complex world of human septins: implications for health and disease. Clin. Genet. 2010; 77(6):511-24. [PMID: 20236126]
- Song K, Russo G, and Krauss M. Septins As Modulators of Endo-Lysosomal Membrane Traffic. Front Cell Dev Biol 2016; 4:124. [PMID: 27857942]
- Bridges AA, and Gladfelter AS. Septin Form and Function at the Cell Cortex. J. Biol. Chem. 2015; 290(28):17173-80. [PMID: 25957401]
- Bridges AA, Jentzsch MS, Oakes PW, Occhipinti P, and Gladfelter AS. Micron-scale plasma membrane curvature is recognized by the septin cytoskeleton. J. Cell Biol. 2016; 213(1):23-32. [PMID: 27044896]
- Lobato-Márquez D, and Mostowy S. Septins recognize micron-scale membrane curvature. J. Cell Biol. 2016; 213(1):5-6. [PMID: 27044893]
- Hu J, Bai X, Bowen JR, Dolat L, Korobova F, Yu W, Baas PW, Svitkina T, Gallo G, and Spiliotis ET. Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Curr. Biol. 2012; 22(12):1109-15. [PMID: 22608511]


