What are Rho GTPases?
The Ras homologous (Rho) protein family is a member of the Ras superfamily of small GTPases. Small GTPases are monomeric proteins and function as molecular switches that turn “on” or turn “off” signal transduction pathways in response to chemical or mechanical stimuli.
Rho GTPases are central regulators of actin reorganization and consequently function in cellular processes such as cell migration, wound healing, cell adhesion, cell polarity, membrane trafficking and cytokinesis (reviewed in [1][2]). Rho GTPases are highly conserved in all eukaryotes studied so far and Rho, Rac and Cdc42 are the most prominent of the 20 members identified. In response to growth factors, Rho promotes the formation of actin stress fibers and focal adhesions [3], Rac regulates the formation of membrane ruffles and lamellipodia [4] and Cdc42 is required for the formation of actin microspikes and filopodium [5][6].
Regulators and Effectors of Rho GTPases
A large number of upstream regulators, categorized as GTP-ase activating proteins (GAPs), Guanine nucleotide exchange factors (GEFs) and Guanine nucleotide dissociation inhibitors (GDIs), regulate Rho GTPases. Being molecular switches, Rho GTPases execute their function by switching from the inactive GDP-bound state to the active GTP-bound state in response to extracellular stimuli. RhoGEFs [7] promote activation of Rho GTPases whereas RhoGAPs [8] and RhoGDIs [9] promote formation of the inactive GDP-bound form. The Rho GTPases are also mechanically regulated. For instance, mechanical activation of Rac1 is required for the force-dependent growth of adherens junctions [10].
Rho GTPases act on over 100 different targets, regulating multiple signal transduction pathways, resulting in diverse cellular functions [11]. Two major effectors of Rho required for assembly of contractile actomyosin rings are mDia and ROCK (Rho-associated kinase) [12]. Rac and Cdc42 activate Arp2/3 complex through WAVE and N-WASP proteins respectively, thereby promoting actin polymerization [13].
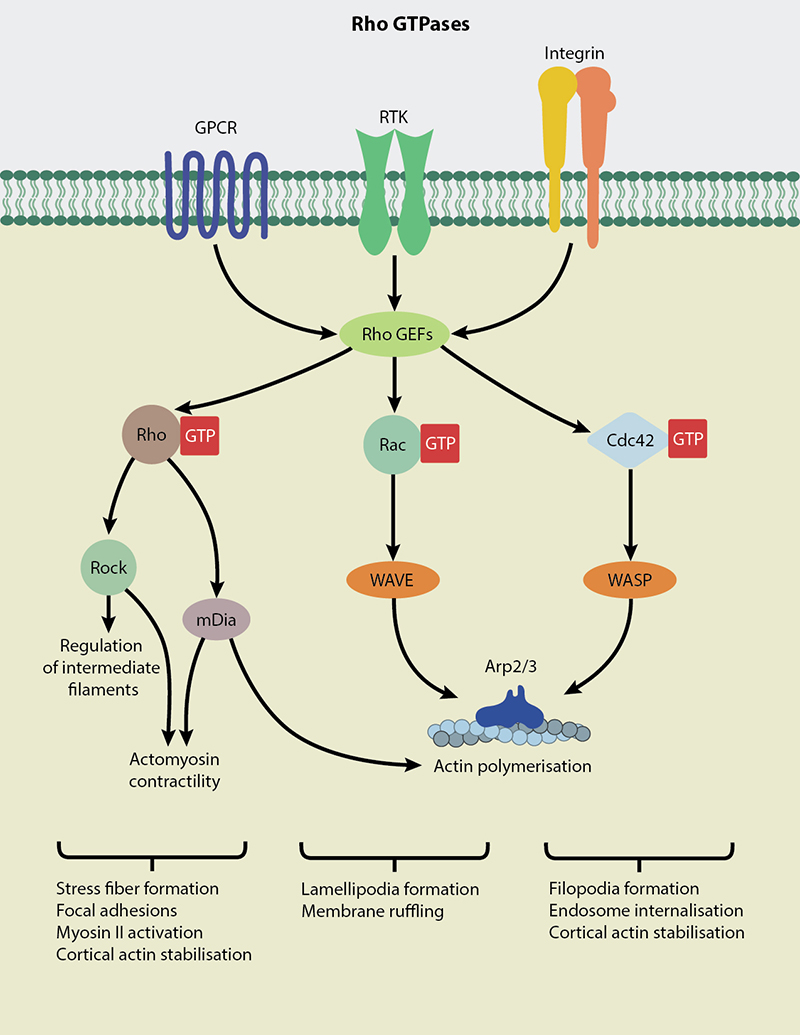
Rho family members are key regulators of actin reorganization and intermediate filaments.
Cellular roles of Rho GTPases
The Rho GTPases play a well-documented role in clathrin-independent endocytosis (reviewed in [14]). RhoA and Rac are involved in a dynamin-dependent pathway, whereas cdc42 is involved in a dynamin-independent pathway known as CLIC/GEEC pathway [15].
Live imaging of Rho GTPases show that during cell migration, both Rho and Rac are active at the leading edge protrusions, contrary to the previous notion of Rac being active at the leading edge and Rho at the retracting edge or rear of the cell [16][17]. Although Rho, Rac and Cdc42 have distinct functions in cell migration and cellular wound repair, cross-talk between the Rho GTPases and the cytoskeleton is critical for these processes [18]. For example, during wound healing, Rho is required for myosin II activation, Rho and Cdc42 for actomyosin ring stabilization and Rac for actin mobilization towards the wound. Cross-talk between the three GTPases is required for organization and translocation of the actomyosin ring [19]. Cross-talk between the Ras superfamily members are also essential for many cellular functions. In Zebrafish liver cancer models, RhoA was shown to suppress Kras- driven liver tumorigenesis [20]. In ovarian cancer, however, a mechanosensitive Rho-ROCK pathway was shown to increase ovarian cancer metastasis on soft tissues [21].
References
- Heasman SJ, and Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008; 9(9):690-701. [PMID: 18719708]
- Hall A. Rho family GTPases. Biochem. Soc. Trans. 2012; 40(6):1378-82. [PMID: 23176484]
- Ridley AJ, and Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992; 70(3):389-99. [PMID: 1643657]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, and Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992; 70(3):401-10. [PMID: 1643658]
- Nobes CD, and Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995; 81(1):53-62. [PMID: 7536630]
- Tapon N, and Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr. Opin. Cell Biol. 1997; 9(1):86-92. [PMID: 9013670]
- Cook DR, Rossman KL, and Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene 2013; 33(31):4021-35. [PMID: 24037532]
- van Buul JD, Geerts D, and Huveneers S. Rho GAPs and GEFs: controling switches in endothelial cell adhesion. Cell Adh Migr 2014; 8(2):108-24. [PMID: 24622613]
- Ota T, Maeda M, Okamoto M, and Tatsuka M. Positive regulation of Rho GTPase activity by RhoGDIs as a result of their direct interaction with GAPs. BMC Syst Biol 2015; 9:3. [PMID: 25628036]
- Collins C, and Tzima E. Rac[e] to the pole: setting up polarity in endothelial cells. Small GTPases 2014; 5:e28650. [PMID: 25202973]
- Bishop AL, and Hall A. Rho GTPases and their effector proteins. Biochem. J. 2000; 348 Pt 2:241-55. [PMID: 10816416]
- Watanabe N, Kato T, Fujita A, Ishizaki T, and Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1999; 1(3):136-43. [PMID: 10559899]
- Spiering D, and Hodgson L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr 2011; 5(2):170-80. [PMID: 21178402]
- Mayor S, Parton RG, and Donaldson JG. Clathrin-independent pathways of endocytosis. Cold Spring Harb Perspect Biol 2014; 6(6). [PMID: 24890511]
- Sandvig K, Pust S, Skotland T, and van Deurs B. Clathrin-independent endocytosis: mechanisms and function. Curr. Opin. Cell Biol. 2011; 23(4):413-20. [PMID: 21466956]
- Hodgson L, Shen F, and Hahn K. Biosensors for characterizing the dynamics of rho family GTPases in living cells. Curr Protoc Cell Biol 2010; Chapter 14:Unit 14.11.1-26. [PMID: 20235099]
- Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, and Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature 2009; 461(7260):99-103. [PMID: 19693013]
- Zegers MM, and Friedl P. Rho GTPases in collective cell migration. Small GTPases 2014; 5:e28997. [PMID: 25054920]
- Abreu-Blanco MT, Verboon JM, and Parkhurst SM. Coordination of Rho family GTPase activities to orchestrate cytoskeleton responses during cell wound repair. Curr. Biol. 2014; 24(2):144-55. [PMID: 24388847]
- Chew TW, Liu XJ, Liu L, Spitsbergen JM, Gong Z, and Low BC. Crosstalk of Ras and Rho: activation of RhoA abates Kras-induced liver tumorigenesis in transgenic zebrafish models. Oncogene 2013; 33(21):2717-27. [PMID: 23812423]
- McGrail DJ, Kieu QMN, and Dawson MR. The malignancy of metastatic ovarian cancer cells is increased on soft matrices through a mechanosensitive Rho-ROCK pathway. J. Cell. Sci. 2014; 127(Pt 12):2621-6. [PMID: 24741068]


