What are Rab GTPases?
The Rab proteins constitute the largest family of small GTPases belonging to the Ras superfamily, with approximately 70 members identified in humans. Rab GTPases are the primary regulators of the vesicular trafficking pathways that are responsible for transporting the vast array of cellular cargo across membrane organelles. The Rabs function as molecular switches in regulating the formation, transport, docking, and fusion of transport vesicles during membrane transport [1][2].
Rab Regulators and Effectors
The subcellular localization and activity of the Rab proteins depend on their nucleotide-bound states and the cycling between activated, membrane-linked Rab-GTP and inactive cytosolic Rab-GDP is regulated by a group of GEFs, GAPs and GDIs. Membrane anchorage occurs via geranylgeranyl groups on the cytoplasmic domain of the Rabs and is facilitated by Rab Escort Proteins (REPs), which present the newly synthesized Rabs to geranyl transferases for prenylation. The membrane specificity for the different Rabs is provided by GDI Displacement Factors (GDFs) that identify distinct Rab-GDI complexes from the cytosol and promote the dissociation of the GDIs, before delivering the Rabs to the membrane [3]. Activated Rabs function by recruiting a distinct set of effectors such as coat proteins [4], cytoskeletal motors [5][6], kinases and phosphatases [7] and membrane fusion proteins [8][9] for mediating the various pathways of membrane transport. Disrupted trafficking resulting from dysfunction of the Rab GTPases, their regulators or effectors is associated with tumorigenesis [10], viral and bacterial infections [11][12] and several inherited disorders of the nervous system, eyes, skin, and bones [13][14][15].
Rab GTPase functions
Cellular communication with the extracellular environment is mediated by the inward and outward trafficking of vesicles during endocytic and exocytic/secretory pathways, as seen in physiological processes such as immune responses, embryonic morphogenesis and endocrine signaling. The directionality and specificity of cargo transport amidst intricately connected pathways is maintained by the association of specific Rabs with distinct intracellular membranes and their transport vesicles.
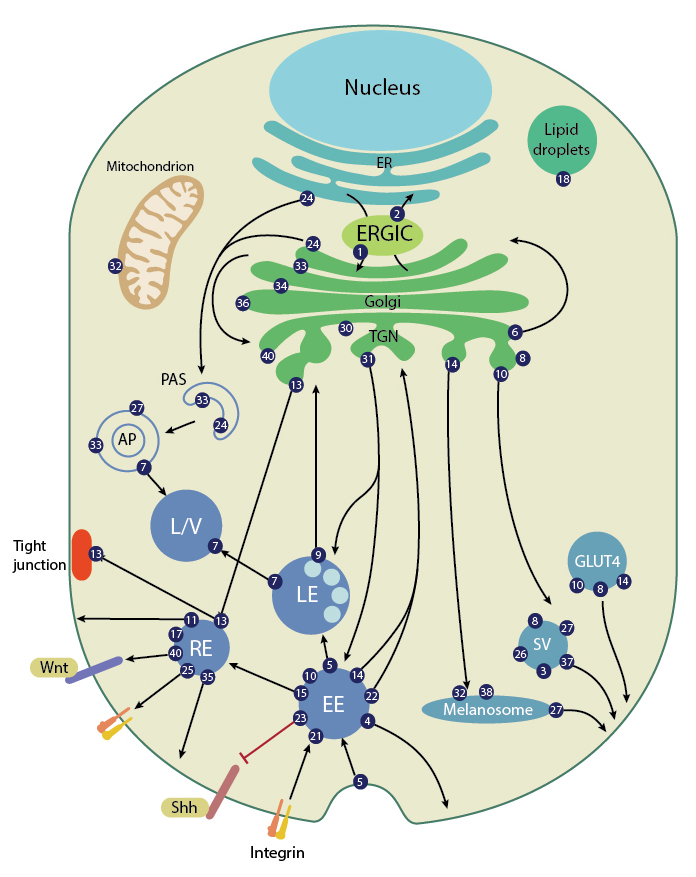
Rab GTPases in endocytosis and exocytosis pathways
Rab GTPases in endocytic pathways
Some of the Rab isoforms commonly identified in the endocytic pathway are Rab4, Rab5, Rab7 and Rab11; Rab4, Rab5 and Rab11 function in the early endocytic pathway, whereas Rab7 and Rab9 regulate the later stages of endocytosis (reviewed in [16]). Rab5 primes the pathway by regulating the formation of coated vesicles, the fusion of vesicles with early endosomes and the homophilic fusion between early endosomes [17]. Endosomal maturation into multivesicular bodies, followed by fusion to lysosomes is controlled by Rab7 GTPase [18], though later studies have suggested a dispensable role for Rab7 in endosomal maturation [19]. Recent work has also associated the Rab7b isoform with later stages of trafficking to the trans-golgi network [20]. Rab4, Rab 11 and Rab9 are the recycling GTPases and mediate retro-transport of the cargo to the plasma membrane and the Golgi complex [21][22]. Rab11-mediated endosomal recycling is shown to be essential for the maintenance of cellular polarity [23].
Rab GTPases in exocytic/secretory pathways
In the presence of external stimuli, the exocytosis of secretory products stored in cell-specific organelles is regulated by Rab GTPases such as Rab3 and Rab27. The localization of Rab27 on secretory lysosomes such as melanosomes in melanocytes, lytic granules in cytotoxic T cells and dense granules in platelets is directly linked to its regulation of melanosome transport and lytic granule exocytosis [24]. In humans, mutations resulting in a loss-of-function in Rab27 is associated with Gricelli syndrome, characterized by partial cutaneous albinism and immunodeficient phenotypes [25][26]. Rab27 is also known to regulate the exocytosis of conventional secretory granules in various endocrine, exocrine and immune cells [27]. The Rab3 isoforms have been identified as regulators of the terminal stages of exocytosis in brain and endocrine cells. In brain cells, Rab3 regulates the late stages of synaptic vesicle fusion and neurotransmitter release [28], while in endocrine cells such as the pancreatic beta cells, Rab3 associates with the secretory granules and facilitates their rapid replenishment into releasable vesicle pools [29].
Rab GTPase in actin remodeling
Outside their canonical role in membrane trafficking, the Rabs function in various cellular processes such as proliferation, adhesion, motility and survival. Rab7b has been specifically associated with RhoA activation and myosin light chain phosphorylation, thereby facilitating cell polarization, adhesion and migration [30]. Recent studies have proposed a role for the early endosome regulator, Rab5, in cell migration that involves Rho GTPase-mediated integrin signaling and focal adhesion disassembly [31].
References
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009; 10(8):513-25. [PMID: 19603039]
- Hutagalung AH, and Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 2011; 91(1):119-49. [PMID: 21248164]
- Sivars U, Aivazian D, and Pfeffer SR. Yip3 catalyses the dissociation of endosomal Rab-GDI complexes. Nature 2003; 425(6960):856-9. [PMID: 14574414]
- Carroll KS, Hanna J, Simon I, Krise J, Barbero P, and Pfeffer SR. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science 2001; 292(5520):1373-6. [PMID: 11359012]
- Roland JT, Kenworthy AK, Peranen J, Caplan S, and Goldenring JR. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol. Biol. Cell 2007; 18(8):2828-37. [PMID: 17507647]
- Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, and Hammer JA. Identification of an organelle receptor for myosin-Va. Nat. Cell Biol. 2002; 4(4):271-8. [PMID: 11887186]
- Shin H, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk MR, Modregger J, Uttenweiler-Joseph S, Wilm M, Nystuen A, Frankel WN, Solimena M, De Camilli P, and Zerial M. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J. Cell Biol. 2005; 170(4):607-18. [PMID: 16103228]
- Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, and Zerial M. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J. Cell Biol. 2000; 151(3):601-12. [PMID: 11062261]
- Simonsen A, Lippé R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, and Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 1998; 394(6692):494-8. [PMID: 9697774]
- Cheng KW, Lahad JP, Kuo W, Lapuk A, Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu KH, Fishman D, Gray JW, and Mills GB. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat. Med. 2004; 10(11):1251-6. [PMID: 15502842]
- Coyne CB, Shen L, Turner JR, and Bergelson JM. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe 2007; 2(3):181-92. [PMID: 18005733]
- Smith AC, Heo WD, Braun V, Jiang X, Macrae C, Casanova JE, Scidmore MA, Grinstein S, Meyer T, and Brumell JH. A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J. Cell Biol. 2007; 176(3):263-8. [PMID: 17261845]
- Seabra MC, Brown MS, and Goldstein JL. Retinal degeneration in choroideremia: deficiency of rab geranylgeranyl transferase. Science 1993; 259(5093):377-81. [PMID: 8380507]
- Aligianis IA, Johnson CA, Gissen P, Chen D, Hampshire D, Hoffmann K, Maina EN, Morgan NV, Tee L, Morton J, Ainsworth JR, Horn D, Rosser E, Cole TRP, Stolte-Dijkstra I, Fieggen K, Clayton-Smith J, Mégarbané A, Shield JP, Newbury-Ecob R, Dobyns WB, Graham JM, Kjaer KW, Warburg M, Bond J, Trembath RC, Harris LW, Takai Y, Mundlos S, Tannahill D, Woods CG, and Maher ER. Mutations of the catalytic subunit of RAB3GAP cause Warburg Micro syndrome. Nat. Genet. 2005; 37(3):221-3. [PMID: 15696165]
- Aligianis IA, Morgan NV, Mione M, Johnson CA, Rosser E, Hennekam RC, Adams G, Trembath RC, Pilz DT, Stoodley N, Moore AT, Wilson S, and Maher ER. Mutation in Rab3 GTPase-activating protein (RAB3GAP) noncatalytic subunit in a kindred with Martsolf syndrome. Am. J. Hum. Genet. 2006; 78(4):702-7. [PMID: 16532399]
- Wandinger-Ness A, and Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 2014; 6(11):a022616. [PMID: 25341920]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, and Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 1992; 70(5):715-28. [PMID: 1516130]
- Rink J, Ghigo E, Kalaidzidis Y, and Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005; 122(5):735-49. [PMID: 16143105]
- Vanlandingham PA, and Ceresa BP. Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. J. Biol. Chem. 2009; 284(18):12110-24. [PMID: 19265192]
- Progida C, Cogli L, Piro F, De Luca A, Bakke O, and Bucci C. Rab7b controls trafficking from endosomes to the TGN. J. Cell. Sci. 2010; 123(Pt 9):1480-91. [PMID: 20375062]
- Arjonen A, Alanko J, Veltel S, and Ivaska J. Distinct recycling of active and inactive β1 integrins. Traffic 2012; 13(4):610-25. [PMID: 22222055]
- Dong B, Kakihara K, Otani T, Wada H, and Hayashi S. Rab9 and retromer regulate retrograde trafficking of luminal protein required for epithelial tube length control. Nat Commun 2013; 4:1358. [PMID: 23322046]
- Jing J, and Prekeris R. Polarized endocytic transport: the roles of Rab11 and Rab11-FIPs in regulating cell polarity. Histol. Histopathol. 2009; 24(9):1171-80. [PMID: 19609864]
- Hume AN, Collinson LM, Rapak A, Gomes AQ, Hopkins CR, and Seabra MC. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 2001; 152(4):795-808. [PMID: 11266470]
- Van Gele M, Dynoodt P, and Lambert J. Griscelli syndrome: a model system to study vesicular trafficking. Pigment Cell Melanoma Res 2009; 22(3):268-82. [PMID: 19243575]
- Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, and Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 2001; 152(4):825-34. [PMID: 11266472]
- Tolmachova T, Anders R, Stinchcombe J, Bossi G, Griffiths GM, Huxley C, and Seabra MC. A general role for Rab27a in secretory cells. Mol. Biol. Cell 2003; 15(1):332-44. [PMID: 14617806]
- Cheng Y, Wang J, Wang Y, and Ding M. Synaptotagmin 1 directs repetitive release by coupling vesicle exocytosis to the Rab3 cycle. Elife 2015; 4. [PMID: 25710274]
- Cazares VA, Subramani A, Saldate JJ, Hoerauf W, and Stuenkel EL. Distinct actions of Rab3 and Rab27 GTPases on late stages of exocytosis of insulin. Traffic 2014; 15(9):997-1015. [PMID: 24909540]
- Borg M, Bakke O, and Progida C. A novel interaction between Rab7b and actomyosin reveals a dual role in intracellular transport and cell migration. J. Cell. Sci. 2014; 127(Pt 22):4927-39. [PMID: 25217632]
- Mendoza P, Díaz J, and Torres VA. On the role of Rab5 in cell migration. Curr. Mol. Med. 2014; 14(2):235-45. [PMID: 24467205]


