What are immunoglobulin superfamily cell adhesion molecules?
Members of the Immunoglobulin superfamily include vascular and neural cell adhesions molecules (VCAM and NCAM), intercellular adhesion molecules (ICAM) and the Nectins and nectin-like (Necl) proteins. Nectins in particular are involved in the formation of cadherin-based cell-cell junctions [1], mediating initial cell-cell contacts via nectin-nectin or nectin-Necl binding and establishing links to the actin cytoskeleton via nectin-afadin binding [2]. Of the four major groups of CAMs, IgCAMs are the only group that function independently of calcium.
The Ig superfamily is a large group of cell surface molecules that includes members such as:
- Vascular cell adhesion molecules (VCAM)
- Neural cell adhesion molecules (NCAM)
- Intercellular adhesion molecules (ICAM)
- Nectin and nectin-like (Nec1) family
Members of the Ig superfamily resemble each other in their three-dimensional structure as well as their amino acid sequence (reviewed in [3]). ICAMs and NCAMs form heterophilic and homophilic interactions (respectively) with adhesion molecules on other cells through a rigid extracytoplasmic rod domain that contains at least one flexible hinge domain [4][5]. Although nectins and Necls form both heterophilic and homophilic interactions (reviewed in [1]), their homophilic interactions tend to be stronger [6].
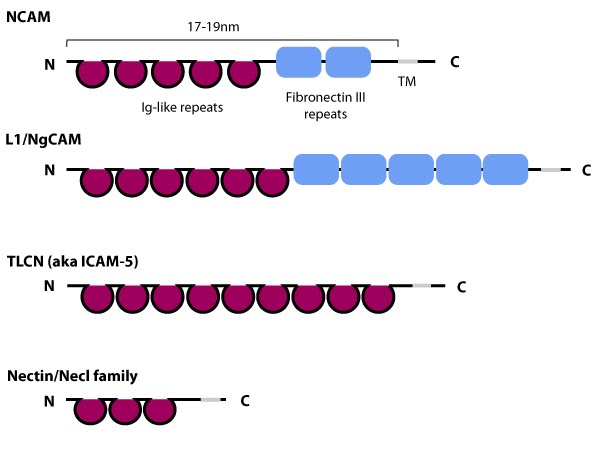
The extracytoplasmic portion of all Ig superfamily members is composed of a variable number of Ig domains (~3.7 nm per Ig domain) that together yield a final length of approximately 17-19 nm for both NCAM [3] and ICAM-1 [2]; certain Ig family members also contain a variable number of fibronectin III repeat domains. The transmembrane domain (TM) located at the extreme carboxy-terminus leaves a relatively small portion on the intracellular side for associating with other proteins. ICAM-1 is similar to ICAM-5 (not shown) but contains five Ig domains. Nectin and Necls share a similar structure, with three extracytoplasmic Ig-like domains (reviewed in [4])
Most ICAMs are expressed mainly by immune cells and endothelial cells, however brain-specific forms also exist (e.g. ICAM-5 aka TLCN) [7][8]. All ICAMs appear to share lymphocyte function-associated antigen-1 (LFA-1, CD11a/CD18, αLβ2 integrin) as their counter receptor [9][10][11]. LFA-1 integrin is found on the surface of leukocytes where it modulates adhesion-dependent events that are essential for immune system activities. In the brain, LFA-1 expression appears to be restricted to resident macrophages (microglia) and its expression is tied to microglia activation [12]. ICAM-1 and LFA-1 binding is magnesium-dependent [9][13] and the sites for LFA-1 binding lie in the first two amino-terminal Ig domains of ICAM-1; the residues involved in binding to LFA-1 are conserved in other ICAMs [4].
Nectin and Necl family
Nectins and nectin-like molecules (Necls) are expressed in a number of cell types where they have been shown to be important for cell-cell adhesion and the formation of stable junctions (e.g adherens junctions). Nectins and Necls also play a role in various cellular activities including cell polarization, migration, growth and cell fate (reviewed in [1][14]). Nectin and Necls interact with and share a number of binding partners through their cytoplasmic domain, however, only nectins bind to afadin, an F-actin binding protein.
References
- Takai Y, and Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J. Cell. Sci. 2003; 116(Pt 1):17-27. [PMID: 12456712]
- Kurita S, Ogita H, and Takai Y. Cooperative role of nectin-nectin and nectin-afadin interactions in formation of nectin-based cell-cell adhesion. J. Biol. Chem. 2011; 286(42):36297-303. [PMID: 21880730]
- Takada Y, Ye X, and Simon S. The integrins. Genome Biol. 2007; 8(5):215. [PMID: 17543136]
- Staunton DE, Dustin ML, Erickson HP, and Springer TA. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell 1990; 61(2):243-54. [PMID: 1970514]
- Becker JW, Erickson HP, Hoffman S, Cunningham BA, and Edelman GM. Topology of cell adhesion molecules. Proc. Natl. Acad. Sci. U.S.A. 1989; 86(3):1088-92. [PMID: 2915974]
- Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, and Takai Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J. Biol. Chem. 2000; 275(14):10291-9. [PMID: 10744716]
- Mori K, Fujita SC, Watanabe Y, Obata K, and Hayaishi O. Telencephalon-specific antigen identified by monoclonal antibody. Proc. Natl. Acad. Sci. U.S.A. 1987; 84(11):3921-5. [PMID: 3295872]
- Yoshihara Y, Oka S, Nemoto Y, Watanabe Y, Nagata S, Kagamiyama H, and Mori K. An ICAM-related neuronal glycoprotein, telencephalin, with brain segment-specific expression. Neuron 1994; 12(3):541-53. [PMID: 7794412]
- Marlin SD, and Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell 1987; 51(5):813-9. [PMID: 3315233]
- Staunton DE, Dustin ML, and Springer TA. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature 1989; 339(6219):61-4. [PMID: 2497351]
- Mizuno T, Yoshihara Y, Kagamiyama H, Ohsawa K, Imai Y, Kohsaka S, and Mori K. Neuronal adhesion molecule telencephalin induces rapid cell spreading of microglia. Brain Res. 1999; 849(1-2):58-66. [PMID: 10592287]
- Moneta ME, Gehrmann J, Töpper R, Banati RB, and Kreutzberg GW. Cell adhesion molecule expression in the regenerating rat facial nucleus. J. Neuroimmunol. 1993; 45(1-2):203-6. [PMID: 8101190]
- Dustin ML, and Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature 1989; 341(6243):619-24. [PMID: 2477710]
- Takai Y, Miyoshi J, Ikeda W, and Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat. Rev. Mol. Cell Biol. 2008; 9(8):603-15. [PMID: 18648374]


