What are cell-matrix adhesions?
The extracellular matrix and the basal lamina
Cell-matrix adhesion is the interaction of a cell with the extracellular matrix, mediated by multi-protein adhesion structures such as focal adhesions, fibrillar adhesions and podosomes.
The ECM is a network of extracellular molecules which are secreted locally to ensure cell and tissue cohesion. The ECM also serves as a reservoir for extracellular signaling molecules that control cell growth, migration, and differentiation. The major classes of ECM molecules are proteoglycans, collagens and multi-adhesive matrix proteins (e.g. laminin, fibronectin). In mammals, the ECM is commonly known as “connective tissue”. ECM components are linked to each other through diverse protein and carbohydrate-binding domains. For stability in tissues, cells are linked to the ECM through cell adhesion receptors (e.g. integrins). A specialized form of extracellular matrix that underlies the basal side of polarized epithelial cell sheets to separate them from the underlying connective tissue is the basal lamina [1].
Basal laminae (plural) also surround individual muscle cells, fat cells, and cells lining peripheral nerve cell axons (i.e. Schwann cells) [1]. The basal lamina is thin and flexible, and is composed of closely packed matrix molecules that lack significant volume. The basal lamina components are synthesized and deposited by the cells on either side: the epithelial cells and the cells within the underlying bed of connective tissue (i.e. fibroblasts). The basal laminae forms a cohesive network and mechanical connection between cells and their external environment. Force-driven signals originating between the basal lamina components (i.e. fibronectin) and linked cell adhesion receptors (i.e. integrins) is communicated to the interior of cells through a mechanotransduction system to influence cell polarity, metabolism, fate, and migration.
The key constituents found in the basal lamina are glycoproteins (i.e. laminin, collagen) and proteoglycans (i.e. perlecan), however, the precise composition varies from tissue to tissue and various other molecules (e.g. fibronectin) can also be found [1].
What are cell-matrix adhesions?
Cell-matrix interactions are mediated by adhesion receptors and lead to the formation of multi-protein adhesion structures that interact with the actin cytoskeleton at the cell interior; collectively, they are called cell-matrix adhesion complexes (CMACs) [2].
These adhesions act as vital information processing centers that enable cells to sense numerous extracellular signals that convey information about the chemistry, geometry, and physical properties of the ECM (reviewed in [3]). The substrate type or chemical composition (reviewed in [4]), its rigidity [5][6], and the surface topography [7][8][9](reviewed in [10][11][12]) influence force-induced events through CMACs, and mechanosensitive cells transmit this information through subsequent mechanotransduction pathways and signaling cascades to influence diverse processes such as the cell shape, polarity, fate, motility and deposition and/or restructuring of ECM components [5][13][14][15] (reviewed in [3][11][16][17]).
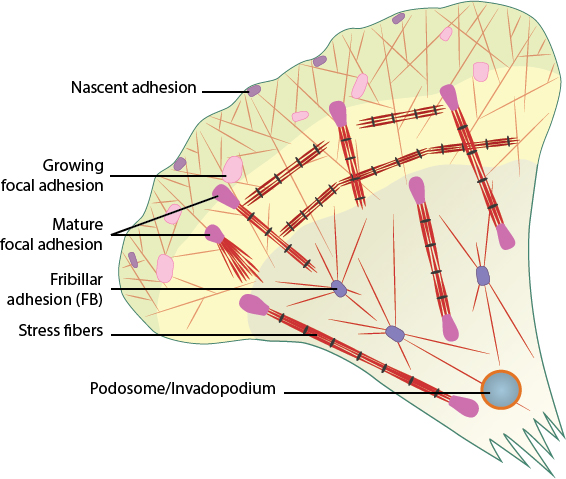
Cell-matrix adhesion complexes include nascent adhesions, focal adhesions (growing and mature), fibrillar adhesions, stress fibers, and podosomes/invadopodia.
References
- Erickson AC, and Couchman JR. Still more complexity in mammalian basement membranes. J. Histochem. Cytochem. 2000; 48(10):1291-306. [PMID: 10990484]
- Lock JG, Wehrle-Haller B, and Strömblad S. Cell-matrix adhesion complexes: master control machinery of cell migration. Semin. Cancer Biol. 2007; 18(1):65-76. [PMID: 18023204]
- Geiger B, Spatz JP, and Bershadsky AD. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009; 10(1):21-33. [PMID: 19197329]
- Hersel U, Dahmen C, and Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003; 24(24):4385-415. [PMID: 12922151]
- Discher DE, Janmey P, and Wang Y. Tissue cells feel and respond to the stiffness of their substrate. Science 2005; 310(5751):1139-43. [PMID: 16293750]
- Engler AJ, Sen S, Sweeney HL, and Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126(4):677-89. [PMID: 16923388]
- Curtis A, and Wilkinson C. New depths in cell behaviour: reactions of cells to nanotopography. Biochem. Soc. Symp. 1999; 65:15-26. [PMID: 10320930]
- Dalby MJ, Riehle MO, Johnstone H, Affrossman S, and Curtis ASG. In vitro reaction of endothelial cells to polymer demixed nanotopography. Biomaterials 2002; 23(14):2945-54. [PMID: 12069336]
- Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, and Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 2002; 16(10):1195-204. [PMID: 12153987]
- Vogel V, and Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006; 7(4):265-75. [PMID: 16607289]
- Curtis A, and Riehle M. Tissue engineering: the biophysical background. Phys Med Biol 2001; 46(4):R47-65. [PMID: 11324976]
- Spatz JP, and Geiger B. Molecular engineering of cellular environments: cell adhesion to nano-digital surfaces. Methods Cell Biol. 2007; 83:89-111. [PMID: 17613306]
- Cavalcanti-Adam EA, Volberg T, Micoulet A, Kessler H, Geiger B, and Spatz JP. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 2007; 92(8):2964-74. [PMID: 17277192]
- Gupton SL, and Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell 2006; 125(7):1361-74. [PMID: 16814721]
- Keren K, Pincus Z, Allen GM, Barnhart EL, Marriott G, Mogilner A, and Theriot JA. Mechanism of shape determination in motile cells. Nature 2008; 453(7194):475-80. [PMID: 18497816]
- Chiquet M, Gelman L, Lutz R, and Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim. Biophys. Acta 2009; 1793(5):911-20. [PMID: 19339214]
- Schwartz MA, and DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr. Opin. Cell Biol. 2008; 20(5):551-6. [PMID: 18583124]


