What are Arf GTPases?
ADP-ribosylation factor (Arf) GTPases are a subfamily of the Ras superfamily of small GTPases. Arf proteins perform diverse and critical functions in fundamental cellular processes like membrane trafficking, lipid modification, cytokinesis and cell adhesion.
Arf GTPases are tightly regulated by specific guanine nucleotide exchange factors (GEFs) with a conserved Sec7 domain, and GTPase-activating proteins (GAPs) with a conserved zinc finger domain. Like all Ras superfamily members, the GEFs switch on/activate Arfs while the GAPS switch off/inactivate them. However the distinguishing feature of Arfs is myristoylation at the N terminus, in addition to the presence of an N-terminal amphipathic helix that gets inserted into the membrane upon GTP binding. Once activated on membranes Arfs recruit their effectors which include coat proteins, lipid modifying enzymes and scaffold proteins (reviewed in [1][2]). The ability of Arfs to recruit a wide variety of effectors is attributed to the multiple membrane-protein contacts made by the Arf/effector complexes [3].
The Arf family of GTPases comprises three different groups of proteins, mainly the Arfs, Arls (Arf-like) and SARs (Secretion-associated and Ras-related proteins)(reviewed in [4]). So far 33 genes encoding the different Arf family members have been reported (http://www.genenames.org/cgi-bin/genefamilies/set/357). There have been over 20 Arl proteins, 2 Sar proteins and 6 mammalian ARF proteins identified to-date.
Cellular roles of Arf GTPases
Arfs are primarily involved in the secretory, endocytic and recycling transport pathways. The Sar1 protein regulates transport of newly synthesized proteins from the endoplasmic reticulum (ER) to the Golgi complex. Sar1 regulates the assembly as well as membrane constriction and scission of COPII coated vesicles from ER [5]. The ARF1 protein functions in retrograde transport from Golgi to the ER through recruitment of COPI coated vesicle proteins [6]. ARF1 also regulates recruitment of clathrin through AP-1, AP-3 and AP-4 complexes as well as monomeric GGA proteins. Indeed, ARF1 has been shown to be indispensable for mouse embryonic development [7]. The ARF6 protein plays a major role in the endocytic pathway impacting cell migration, cell division and homeostasis [8].
Other ARF proteins like ARF3, ARF4 and ARF5 also function at the Golgi and are found to function in pair with ARF1. The ARF1-ARF4 pair regulates retrograde transport from endosomes to the trans-golgi network (TGN), whereas the ARF1-ARF3 pair is required for the transferrin recycling pathway from endosomes to the plasma membrane [9]. The Arl1p interacts with a guanine nucleotide exchange factor Gea2p and a flippase Drs2p to facilitate spatial modulation of membrane organization at the trans-Golgi network [10]. All Arf proteins can stimulate the activity of lipid modifying enzymes like PIP5K and Phospholipase D. Arfs regulate the actin cytoskeleton, primarily through PIP5K and function at multiple stages of cytokinesis (reviewed in [1]). ARF proteins also function in the non-vesicular transport of lipids between organelles [11].
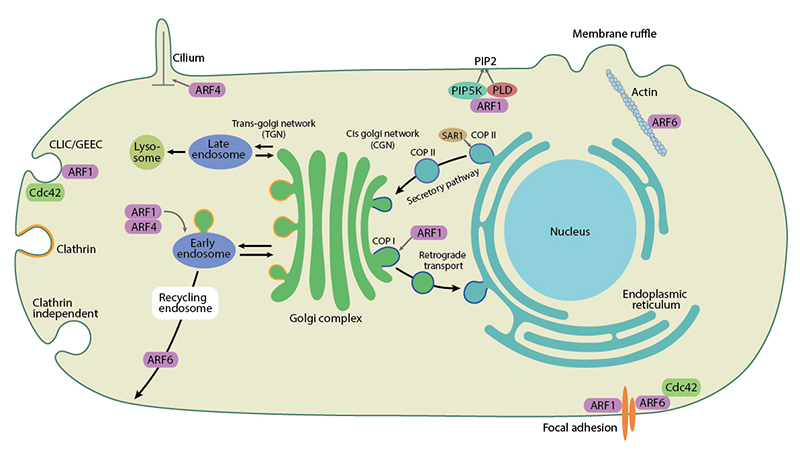
As well as regulating membrane trafficking, Arf family members are also involved in actin remodelling and cell adhesion.
References
- Jackson CL, and Bouvet S. Arfs at a glance. J. Cell. Sci. 2014; 127(Pt 19):4103-9. [PMID: 25146395]
- Donaldson JG, and Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 2011; 12(6):362-75. [PMID: 21587297]
- Cherfils J. Arf GTPases and their effectors: assembling multivalent membrane-binding platforms. Curr. Opin. Struct. Biol. 2014; 29:67-76. [PMID: 25460270]
- Kahn RA, Cherfils J, Elias M, Lovering RC, Munro S, and Schurmann A. Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL, and SAR proteins. J. Cell Biol. 2006; 172(5):645-50. [PMID: 16505163]
- Hariri H, Bhattacharya N, Johnson K, Noble AJ, and Stagg SM. Insights into the mechanisms of membrane curvature and vesicle scission by the small GTPase Sar1 in the early secretory pathway. J. Mol. Biol. 2014; 426(22):3811-26. [PMID: 25193674]
- Beck R, Rawet M, Ravet M, Wieland FT, and Cassel D. The COPI system: molecular mechanisms and function. FEBS Lett. 2009; 583(17):2701-9. [PMID: 19631211]
- Hayakawa N, Ogoh H, Sumiyoshi M, Matsui Y, Nishikawa S, Miyamoto K, Maede Y, Kiyonari H, Suzuki M, and Watanabe T. The ADP-ribosylation factor 1 gene is indispensable for mouse embryonic development after implantation. Biochem. Biophys. Res. Commun. 2014; 453(4):748-53. [PMID: 25305484]
- Schweitzer JK, Sedgwick AE, and D’Souza-Schorey C. ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis. Semin. Cell Dev. Biol. 2010; 22(1):39-47. [PMID: 20837153]
- Nakai W, Kondo Y, Saitoh A, Naito T, Nakayama K, and Shin H. ARF1 and ARF4 regulate recycling endosomal morphology and retrograde transport from endosomes to the Golgi apparatus. Mol. Biol. Cell 2013; 24(16):2570-81. [PMID: 23783033]
- Tsai P, Hsu J, Liu Y, Chen K, and Lee FS. Arl1p regulates spatial membrane organization at the trans-Golgi network through interaction with Arf-GEF Gea2p and flippase Drs2p. Proc. Natl. Acad. Sci. U.S.A. 2013; 110(8):E668-77. [PMID: 23345439]
- Stefan CJ, Manford AG, and Emr SD. ER-PM connections: sites of information transfer and inter-organelle communication. Curr. Opin. Cell Biol. 2013; 25(4):434-42. [PMID: 23522446]


