I-BAR and Other Proteins/Factors
Proteins containing I-BAR (inverted Bin/amphiphysin/Rvs i.e. IRSp53 Missing-in-metastasis homology Domain or IMD) cooperate with various components of actin filament assembly, to promote filopodia protrusion, via several mechanisms including the stimulation of F-actin crosslinking [1].
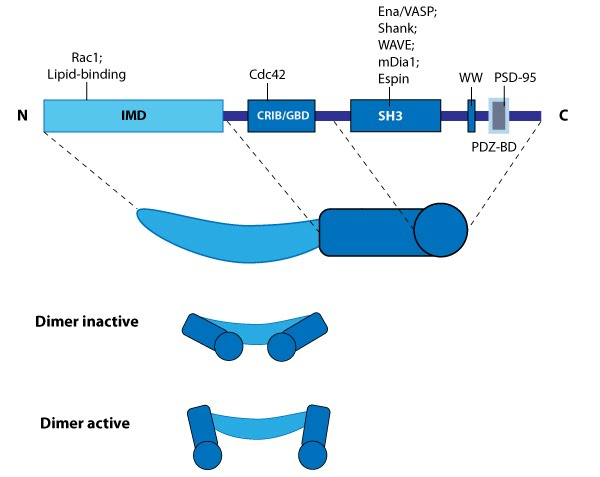
This schematic diagram illustrates the molecular organization of IRSp53 and provides examples for how IRSp53 is represented in figures throughout this resource.
A specific example of an I-BAR domain-containing actin crosslinker and scaffolding protein is IRSp53, which localizes to the tips of filopodia [2]. The activity of IRSp53 is enhanced by Cdc42 or Rac1 GTPases during filopodia formation [3][4][5]. Although this protein performs several functions and binds other actin regulators such as Mena (a Ena/VASP family protein [4]) and formin (e.g. mDia1 [6]), its role in F-actin binding and crosslinking is well established and has been attributed to its IMD domain [5][7].
Additional molecules contribute to the cross linking of actin filaments and these include fimbrin as well as lesser known actin crosslinking proteins such as Arg (Abl-related gene). This latter example is involved in lamellipodial protrusion, independent of its kinase activity, but concomitant with its microtubule binding activity [8].
References
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJG, Evans PR, and McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 2003; 303(5657):495-9. [PMID: 14645856]
- Nakagawa H, Miki H, Nozumi M, Takenawa T, Miyamoto S, Wehland J, and Small JV. IRSp53 is colocalised with WAVE2 at the tips of protruding lamellipodia and filopodia independently of Mena. J. Cell. Sci. 2003; 116(Pt 12):2577-83. [PMID: 12734400]
- Govind S, Kozma R, Monfries C, Lim L, and Ahmed S. Cdc42Hs facilitates cytoskeletal reorganization and neurite outgrowth by localizing the 58-kD insulin receptor substrate to filamentous actin. J. Cell Biol. 2001; 152(3):579-94. [PMID: 11157984]
- Krugmann S, Jordens I, Gevaert K, Driessens M, Vandekerckhove J, and Hall A. Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr. Biol. 2001; 11(21):1645-55. [PMID: 11696321]
- Yamagishi A, Masuda M, Ohki T, Onishi H, and Mochizuki N. A novel actin bundling/filopodium-forming domain conserved in insulin receptor tyrosine kinase substrate p53 and missing in metastasis protein. J. Biol. Chem. 2004; 279(15):14929-36. [PMID: 14752106]
- Fujiwara T, Mammoto A, Kim Y, and Takai Y. Rho small G-protein-dependent binding of mDia to an Src homology 3 domain-containing IRSp53/BAIAP2. Biochem. Biophys. Res. Commun. 2000; 271(3):626-9. [PMID: 10814512]
- Millard TH, Bompard G, Heung MY, Dafforn TR, Scott DJ, Machesky LM, and Fütterer K. Structural basis of filopodia formation induced by the IRSp53/MIM homology domain of human IRSp53. EMBO J. 2005; 24(2):240-50. [PMID: 15635447]
- Miller AL, Wang Y, Mooseker MS, and Koleske AJ. The Abl-related gene (Arg) requires its F-actin-microtubule cross-linking activity to regulate lamellipodial dynamics during fibroblast adhesion. J. Cell Biol. 2004; 165(3):407-19. [PMID: 15138293]


