How is microtubule assembly/disassembly regulated?
GTP hydrolysis has been shown to be a key regulator of microtubule polymerization dynamics. Although the exact mechanisms are poorly understood, two opposing models have been proposed to describe how GTP could alter the conformation of α-tubulin/β-tubulin from the intrinsically ‘bent spring’ shape that resists straightening, to the straight conformation that gives rise to microtubule stability. These models are known as the allosteric model and the lattice model [1].The allosteric model, which is largely supported by electron microscopy studies [2][3] suggests that GTP binding allows the tubulin dimer to adopt a polymerization-competent structure [1]. The lattice model on the other hand suggests that conformational changes occur only after the dimer is incorporated into the growing lattice.
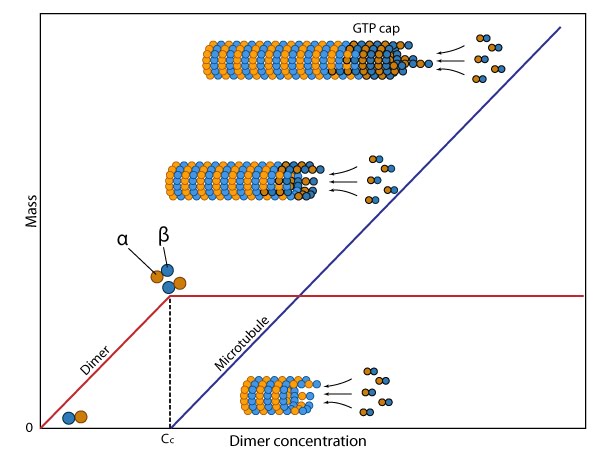
The critical concentration (Cc) marks the level at which alpha and beta tubulin dimers are in equilibrium with microtubules.
Crystallographic studies of α/β-tubulin have provided evidence favoring the lattice model by showing that unpolymerized tubulin dimers adopt a different structure from polymerized tubulin regardless of the nucleotide bound. For example, heterodimer curvature was shown to result from similar domain rearrangements in both α- and β- tubulin despite GTP being bound to only α-tubulin [4]. Moreover the crystal structure of γ-tubulin bound to GTP has been shown to nonetheless take on a curved domain rearrangement [5]. Based on the lattice model, it appears that GTP-binding strengthens longitudinal contacts between neighbouring subunits and provides sufficient binding energy to recruit and straighten the unpolymerized, curved dimer within the lattice [1]. The energy released from GTP hydrolysis can also be stored within the lattice and can be used to perform work if a structure is attached to the microtubule.
References
- Rice LM, Montabana EA, and Agard DA. The lattice as allosteric effector: structural studies of alphabeta- and gamma-tubulin clarify the role of GTP in microtubule assembly. Proc. Natl. Acad. Sci. U.S.A. 2008; 105(14):5378-83. [PMID: 18388201]
- Wang H, and Nogales E. Nucleotide-dependent bending flexibility of tubulin regulates microtubule assembly. Nature 2005; 435(7044):911-5. [PMID: 15959508]
- Buey RM, Díaz JF, and Andreu JM. The nucleotide switch of tubulin and microtubule assembly: a polymerization-driven structural change. Biochemistry 2006; 45(19):5933-8. [PMID: 16681364]
- Ravelli RBG, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, and Knossow M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004; 428(6979):198-202. [PMID: 15014504]
- Aldaz H, Rice LM, Stearns T, and Agard DA. Insights into microtubule nucleation from the crystal structure of human gamma-tubulin. Nature 2005; 435(7041):523-7. [PMID: 15917813]


