How does the tubulin complex assemble?
Microtubules are made up of repeating units of α/β- tubulin heterodimers, which are assembled on a γ-tubulin ring complex (a complex of γ-tubulin and other protein components), during the nucleation phase.
Tubulin
Tubulin is a small globular protein found in all eukaryotic cells. The tubulin family represents about 3-4% of the total protein content in a cell and its members include α-, β-, γ-, δ-, ε-, and ζ-tubulin [1]. Although the different forms of tubulin are similar, they can have different cellular locations and functions. It should be noted that although both actin and tubulin are basic components of the cytoskeleton and possess the ability to hydrolyze NTP, they are evolutionarily distinct with actin being related in structure to hexokinase and tubulins being distantly related to heterotrimeric G-proteins and other GTPases such as Ras [2].
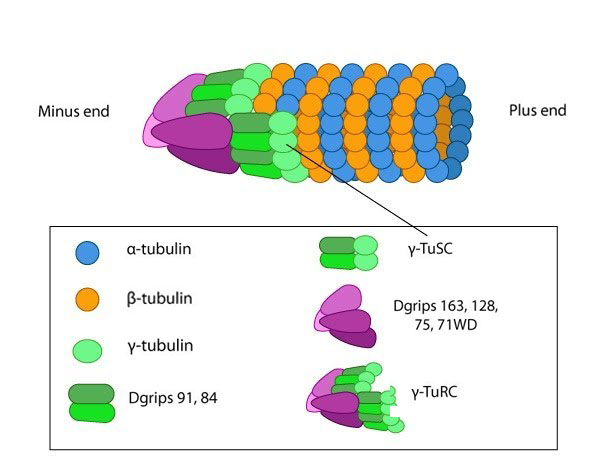
The γ-tubulin ring complex is the starting point for microtubule nucleation.
Repeating units of ~110kDa α-tubulin/β-tubulin heterodimers are the primary component of microtubules. Microtubule assembly starts with the assembly of a tubulin complex. Here, γ-tubulin and other protein components form the γ-tubulin ring complex (γ-TuRC) [2]. The specific cellular roles for additional tubulins such as δ-, ε- and ζ- tubulins are less well known, but they can be found in centrioles and may be involved during mitotic spindle assembly and cell division [3]. Post-translational modification of tubulin such as acetylation, detyrosination, phosphorylation etc. have also been described at length [4]. These modifications may influence the structural properties of microtubules, alter their dynamics and affect how they function within the cell.
Nucleation of Tubulin subunits
Microtubule nucleation is unfavorable under normal conditions found in most living cells. Consequently, microtubules are nucleated from a complex of γ-tubulin and other protein components known as the γ-tubulin ring complex (γ-TuRC). γ-TuRC nucleates and caps the minus end of new filaments by providing stable binding sites for tubulin dimers [5]. Tubulin dimers primarily use longitudinal interactions to bind to each other and to γ−TuRC during the nucleation phase. As the protofilament length increases, lateral interactions between the protofilaments create additional stability that leads to a closed microtubule [2].
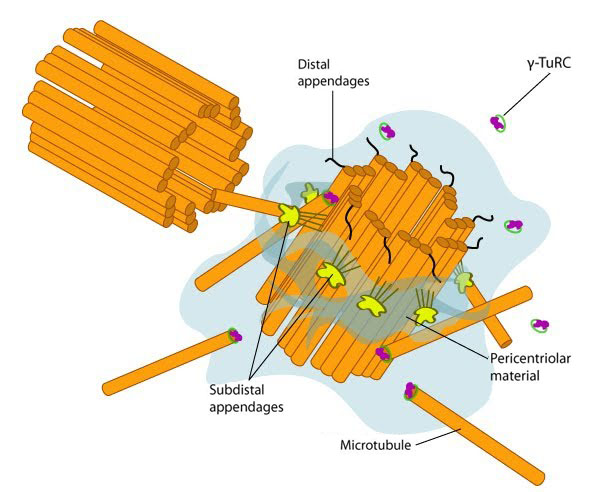
In non-dividing cells, the centrosome, which is also known as the MTOC, consists of a pair of L-shaped centrioles and associated pericentriolar material. The ‘older’ of the two centrioles has additional proteins that form appendages along the exterior surface. The pericentriolar material contains numerous γ-TuRCs that nucleate the microtubule array. The centrioles have microtubules organized in a structure similar to the basal bodies found at the base of cilia and flagella. Figure adapted from [10854327]
References
- Oakley BR. An abundance of tubulins. Trends Cell Biol. 2000; 10(12):537-42. [PMID: 11121746]
- Kollman JM, Merdes A, Mourey L, and Agard DA. Microtubule nucleation by γ-tubulin complexes. Nat. Rev. Mol. Cell Biol. 2011; 12(11):709-21. [PMID: 21993292]
- McKean PG, Vaughan S, and Gull K. The extended tubulin superfamily. J. Cell. Sci. 2001; 114(Pt 15):2723-33. [PMID: 11683407]
- Janke C, and Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2011; 12(12):773-86. [PMID: 22086369]
- Wiese C, and Zheng Y. A new function for the gamma-tubulin ring complex as a microtubule minus-end cap. Nat. Cell Biol. 2000; 2(6):358-64. [PMID: 10854327]


