How does the clathrin coated pit form?
Adaptor proteins such as AP-2, AP180 and CALM (Clathrin-assembly lymphoid myeloid leukaemia protein), which accumulate within the lipid bilayer, are responsible for the recruitment of the triskelion shaped Clathrin trimer. This trimer does not interact with the membrane directly but instead forms a reinforcing lattice structure that acts as a mold in which membrane vesicles may develop. Its influence on membrane curvature is via the adapter proteins which are anchored to the lipid bilayer. Importantly, the adaptor proteins also participate directly in membrane bending and vesicle size determination [1].
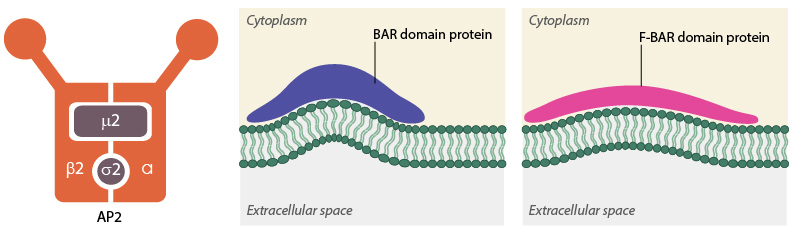
AP2 and BAR-domain proteins
The formation of clathrin-coated pits (CCPs) requires various actin-binding proteins such as those belonging to the BAR (Bin/Amphiphysin/Rvs) superfamily. These include amphiphysin and endophilin in mammals and Rvs161p and Rvs167p in yeast [2]. The role of these BAR proteins is in membrane deformation, essentially promoting its tubulation. By binding to negatively charged membranes, a positive curvature is obtained that follows the concave topology of the protein’s amphipathic α -helix dimer [3][4]. F-BAR proteins, which belong to a sub-family of the BAR superfamily, possess a larger domain that is also concave in shape, yet shallower in its curvature. These proteins are proposed to generate vesicles with a larger radius compared to those proteins with possessing a BAR domain [5][6]. In both cases the proteins may act as curvature sensors that reform the membrane into a shape to which they can readily bind [7]. In the case of clathrin mediated endocytosis, the F-BAR proteins are believed to arrive at the site of clathrin-coated pit formation, before the BAR proteins, and as such may also be involved in nucleation of the CCP [8].
While the BAR domain proteins facilitate tubulation of the membrane, the adaptor proteins including AP-2 or those that possess the epsin N-terminal homology (ENTH) domain such as epsin [9], or the AP-180 N-terminal homology (ANTH) domains such as AP-180 continue to recruit the clathrin triskelion and other regulatory proteins required for the later stages of clathrin-coated vesicle (CCV) formation. Both the ENTH and ANTH domains are highly homologous and bind inositol phospholipids; especially PIP2 [10]. Although both protein subclasses stimulate the formation of a clathrin triskelia network, only proteins possessing the ENTH domain influence membrane curvature, with the clathrin lattice produced by AP-180 stimulation having been shown to remain flat [36]. This influence from the ENTH domains is believed to result from the formation of an additional α –helix ‘α0’ between the ENTH domain and the PIP molecule [11]. It has been proposed that insertion of this domain between the lipid heads of the membrane bilayer may be sufficient to alter membrane curvature alone; however it may also be a synergistic response with clathrin assembly [10].
References
- Schmid EM, and McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature 2007; 448(7156):883-8. [PMID: 17713526]
- Sivadon P, Bauer F, Aigle M, and Crouzet M. Actin cytoskeleton and budding pattern are altered in the yeast rvs161 mutant: the Rvs161 protein shares common domains with the brain protein amphiphysin. Mol. Gen. Genet. 1995; 246(4):485-95. [PMID: 7891662]
- Gallop JL, Jao CC, Kent HM, Butler PJG, Evans PR, Langen R, and McMahon HT. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 2006; 25(12):2898-910. [PMID: 16763559]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJG, Evans PR, and McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 2003; 303(5657):495-9. [PMID: 14645856]
- Shimada A, Niwa H, Tsujita K, Suetsugu S, Nitta K, Hanawa-Suetsugu K, Akasaka R, Nishino Y, Toyama M, Chen L, Liu Z, Wang B, Yamamoto M, Terada T, Miyazawa A, Tanaka A, Sugano S, Shirouzu M, Nagayama K, Takenawa T, and Yokoyama S. Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell 2007; 129(4):761-72. [PMID: 17512409]
- Henne WM, Kent HM, Ford MGJ, Hegde BG, Daumke O, Butler PJG, Mittal R, Langen R, Evans PR, and McMahon HT. Structure and analysis of FCHo2 F-BAR domain: a dimerizing and membrane recruitment module that effects membrane curvature. Structure 2007; 15(7):839-52. [PMID: 17540576]
- Liu J, Sun Y, Drubin DG, and Oster GF. The mechanochemistry of endocytosis. PLoS Biol. 2009; 7(9):e1000204. [PMID: 19787029]
- Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, and McMahon HT. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science 2010; 328(5983):1281-4. [PMID: 20448150]
- Verstreken P, Kjaerulff O, Lloyd TE, Atkinson R, Zhou Y, Meinertzhagen IA, and Bellen HJ. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell 2002; 109(1):101-12. [PMID: 11955450]
- Legendre-Guillemin V, Wasiak S, Hussain NK, Angers A, and McPherson PS. ENTH/ANTH proteins and clathrin-mediated membrane budding. J. Cell. Sci. 2004; 117(Pt 1):9-18. [PMID: 14657269]
- Ford MGJ, Mills IG, Peter BJ, Vallis Y, Praefcke GJK, Evans PR, and McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature 2002; 419(6905):361-6. [PMID: 12353027]


