How does invagination and maturation of the clathrin-coated vesicle occur?
Clathrin-coated vesicle maturation incorporates the activities of a range of proteins. Actin, myosin and WASP all have important roles in the formation and stabilization of clathrin-coated pits (CCPs) [1][2][3]. Evidence also suggests that F-actin influences the lateral movement of CCPs, which effects the subsequent growth of the vesicle [1].
Some proteins act directly on the membrane or clathrin coat whilst others contribute indirectly through regulatory roles or via the adaptor proteins. One protein with a major role in CCP maturation is dynamin, a GTPase that also facilitates scission of the vesicle from the plasma membrane [4]. Although there is no clear involvement of dynamin in yeast, the yeast dynamin-like protein Vps1 has been implicated in endocytosis in this model organism [5].
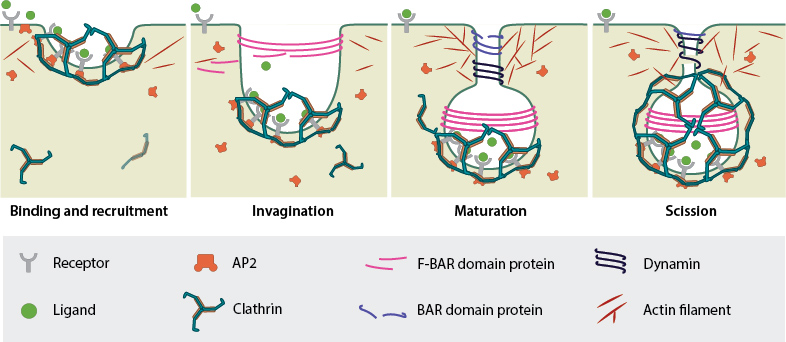
CME starts with receptor binding and recruitment of clathrin for the clathrin coated pit. This is followed by actin cytoskeleton and membrane curvature reorganization for invagination and maturation of the vesicle. Finally, dynamin mediated twisting promotes scission and release of the vesicle.
Dynamin is a non-classical GTPase, with a low affinity to GTP, but a high rate of GTP hydrolysis and GDP dissociation [6]. It is suggested to detect the progression of invagination and therefore act as a sensor for the maturation of the CCP to a CCV [4]. This is facilitated by its unique, non-classical GTPase properties, which best fit the function of dynamin to a two-step process of invagination: 1) the pre-collar rate limiting stage and 2) the post-collar fast assembly stage stimulated by GTP hydrolysis [7]. The second stage is crucial for the scission of clathrin-coated vesicles. It has been proposed that dynamin controls the progress of CCV through a checkpoint system which may either lead to CCV maturation or abortion [4]. To initiate abortion, dynamin will bind auxilin and Hsc70, which will in turn begin disassembly of the clathrin coat, a function normally carried out after scission of the mature vesicle [8]. Similarly, progression through to maturation is governed by dynamin’s recruitment of various accessory proteins that monitor and control membrane curvature (amphiphysin/endophilin) or cargo (SNX9, grb2, TTP) [4].
As mentioned, various nucleation promotion factors including N-WASP, Arp2/3 and cortactin are also recruited to the endocytic site and promote actin polymerization during CCP maturation. These have been shown to co-localize with clathrin at the endocytic site [9][10][11] and are indicative of a role for actin cytoskeleton dyamics in clathrin-coated vesicle formation. Although this is well established in yeast cells [12][13], the influence of the actin cytoskeleton on CCV maturation in mammalian cells is controversial.
In support of a correlation between actin cytoskeleton dynamics and CCV maturation, cortactin was shown to bind to dynamin-2 through its Src-homology 3 (SH3) domain [11][14] and it was also reported that actin arrives at the endocytic site following a burst of dynamin recruitment [15]. As dynamin is primarily active in the later stages of CCV maturation, just prior to and during scission, it was proposed that actin’s influence is particularly prominent in the later stages of clathrin-mediated endocytosis [15][13].
Other studies however suggest the influence of actin cytoskeleton dynamics lies in scission of the vesicle, and not maturation or formation of the CCV. This was suggested when vesicle scission was reported to coincide with the peak level of Arp2/3 mediated actin polymerization [3] and when an 82% reduction in CCV scission was reported following latrunculin B treatment of Swiss 3T3 cells [3]. Although this latter study failed to assess CCV formation, their results were supported by latrunculin A and jasplakinalide treatment of the same cell line. Here, an increase in the number of invaginated CCPs was detected indicating the drugs only inhibited CME at the scission stage [1].
It may be the case that an intact cytoskeleton at the endocytic site is not mandatory and its contribution to CCV formation and maturation will depend on the cell type and environment, as was suggested by a study that considered the importance of an intact actin cytoskeleton by inducing its depolymerization, or arresting its polymerization, whilst monitoring the process of clathrin-mediated endocytosis [4]. Alternative roles for the actin cytoskeleton were proposed when an increase in tubule growth was observed in COS-7 cells following treatment with Latrunculin B. It was concluded here that under normal circumstances, the actin cytoskeleton, along with dynamin, regulate tubule growth by maintaining membrane rigidity [16].
References
- Yarar D, Waterman-Storer CM, and Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol. Biol. Cell 2004; 16(2):964-75. [PMID: 15601897]
- Kaksonen M, Toret CP, and Drubin DG. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 2005; 123(2):305-20. [PMID: 16239147]
- Merrifield CJ, Perrais D, and Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 2005; 121(4):593-606. [PMID: 15907472]
- Loerke D, Mettlen M, Yarar D, Jaqaman K, Jaqaman H, Danuser G, and Schmid SL. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 2009; 7(3):e57. [PMID: 19296720]
- Smaczynska-de Rooij II, Allwood EG, Aghamohammadzadeh S, Hettema EH, Goldberg MW, and Ayscough KR. A role for the dynamin-like protein Vps1 during endocytosis in yeast. J. Cell. Sci. 2010; 123(Pt 20):3496-506. [PMID: 20841380]
- Binns DD, Barylko B, Grichine N, Atkinson MA, Helms MK, Jameson DM, Eccleston JF, and Albanesi JP. Correlation between self-association modes and GTPase activation of dynamin. J. Protein Chem. 1999; 18(3):277-90. [PMID: 10395446]
- Narayanan R, Leonard M, Song BD, Schmid SL, and Ramaswami M. An internal GAP domain negatively regulates presynaptic dynamin in vivo: a two-step model for dynamin function. J. Cell Biol. 2005; 169(1):117-26. [PMID: 15824135]
- Newmyer SL, Christensen A, and Sever S. Auxilin-dynamin interactions link the uncoating ATPase chaperone machinery with vesicle formation. Dev. Cell 2003; 4(6):929-40. [PMID: 12791276]
- Merrifield CJ, Qualmann B, Kessels MM, and Almers W. Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur. J. Cell Biol. 2004; 83(1):13-8. [PMID: 15085951]
- Cao H, Orth JD, Chen J, Weller SG, Heuser JE, and McNiven MA. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol. Cell. Biol. 2003; 23(6):2162-70. [PMID: 12612086]
- Engqvist-Goldstein AEY, and Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 2003; 19:287-332. [PMID: 14570572]
- Robertson AS, Smythe E, and Ayscough KR. Functions of actin in endocytosis. Cell. Mol. Life Sci. 2009; 66(13):2049-65. [PMID: 19290477]
- Saarikangas J, Zhao H, and Lappalainen P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol. Rev. 2010; 90(1):259-89. [PMID: 20086078]
- McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, and Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell Biol. 2000; 151(1):187-98. [PMID: 11018064]
- Merrifield CJ, Feldman ME, Wan L, and Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 2002; 4(9):691-8. [PMID: 12198492]
- Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, and De Camilli P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev. Cell 2005; 9(6):791-804. [PMID: 16326391]


