How does cross-linking of actin filaments aid in filopodia extension?
Once nucleation has taken place, actin filaments begin to extend. This process is primarily facilitated by members of the formin family of proteins, however numerous other proteins also play important roles. In the ‘convergent elongation model’ filament barbed ends are locally associated with each other and must be protected from capping in order for extension to occur [1]. This protection is provided by the activity of proteins such as the Ena/VASP family of proteins which is delivered to the filopodia tip by myosin X [2]. Here, Ena/VASP enhances filament polymerization, and promotes F-actin bundling, thereby stimulating filopodial protrusion [1][3][4][5][6].
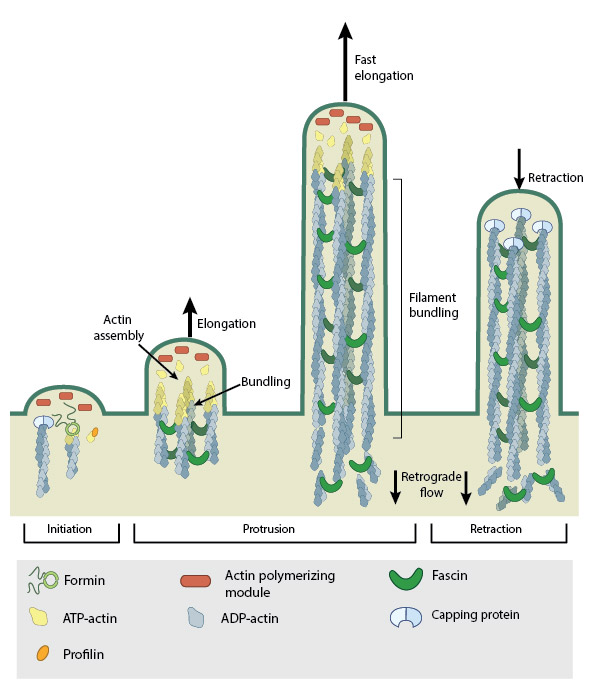
Steps in filopodium formation. Actin filament assembly can be initiated by uncapping pre-formed actin filaments or by de novo formation (which includes both formin- and Arp2/3-mediated [not shown] nucleation). The force produced by actin assembly at the barbed end of actin filaments drives membrane protrusion. Numerous proteins (including IRSp53, Ena/VASP proteins, WASp/Scar proteins) cooperate to promote actin-assembly and enhance bundling of actin filaments by fascin. When the barbed end of the filament is capped, this stops filament assembly and protrusion; continued retrograde movement of the filaments results in retraction of the filopodium. (Figure adapted from Faix J and Rottner K, 2006)
Cross linking strengthens the actin cytoskeleton
As actin filaments extend, force is exerted on the cellular membrane, leading to filopodia protrusion. For efficient protrusion, membrane curvature rigidity must be overcome. This is aided by actin filament cross-linking, which gives the structure the strength required to push against the compressive force of the plasma membrane [6][9]. In nerve growth cones, more than 15 parallel filaments may be bundled together by crosslinking [10]. Bundle stiffness increases with the number of bundled filaments and so contributes to the overall length of the filopodium [6]. Filament bundling results from crosslinking proteins, many of which co-localize at the base of filopodia and work in concert to produce crosslinked filaments [11]. Examples include α-actinin and fascin. The filamin family of proteins are also crucial actin crosslinking and scaffolding proteins and bind to both actin and a number of signaling molecules, including Rho GTPases. Crosslinking also increases the ATPase activity of myosins and increases the tension on filaments [12].
Myosin-X in filament bundling
As well as mediating cargo transport along actin filament bundles, recent studies have implicated myosin-X as integral to the initiation of filopodia and the elongation of long filopodia. This has been attributed to a mechanism of myosin-X that promotes actin filament bundling, similar to cross-linking.
Studying the localization and motility of single myosin-X molecules using TIRF microscopy, Watanabe et al, hypothesized that binding of cargo, and the preceding dimerization of myosin-X monomers at the cell periphery is important for filopodia initiation as it promotes actin filament bundling [13]. This was proposed to occur in a similar manner to regular crosslinking. Previous reports had indicated that even without the cargo-binding FERM domain, lateral movement of myosin-X along the leading edge of lamellipodia promoted actin reorganization and through its motor activity, filopodia initiation. In this case the length of the head and neck domain of myosin-X was proposed to be important for initiation. In the study by Watanbe et al a rapid increase in the rate of recruitment and assembly of myosin-X at filopodia initiation sites moments before protrusion commenced was observed and again this was shown to be independent of the presence of the FERM domain.
After removing the FERM domain from myosin-X (using a FERM domain-truncated construct called M10-ΔFERM) the protein was still able to walk along actin filament bundles and continued to localize at both the leading edge and filopodia tip. Significant changes to the length and stability of the growing filopodia were however noted. Not only were filopodia significantly shorter and more unstable, as previously reported [14][15], but the phased-extension mechanism of elongation observed when complete myosin-X was present, did not occur.
Although it was noted that without the FERM domain the transport of essential cargo to the tips of the filopodia would be insufficient to permit continued elongation, it was also proposed that through FERM-β-integrin interactions at the tips of filopodia (when filopodia are attached to substrates via focal adhesion sites), myosin-X may also possess adhesive qualities. In this hypothesis, as filopodia enter the retraction phase and actin filaments move back towards the cell body by retrograde flow, myosin-X remains at the tips of filopodia together with the adhesion complex. Upon shrinkage of the tips, the protein will rebind to actin filament bundles and allow a new phase of extension to begin from adhesion sites. This mechanism of phased-extension is supported by observations that without the FERM domain myosin-X diffuses back into the cell body during the retraction phase whilst intact myosin-X remains at the tips [13].
How quickly can filopodia extend?
The extension rate of a filopodium will differ depending on the cell type. In each case however, this rate is controlled by the availability of G-actin-ATP, associated structural components and the energetics of membrane bending. The growth of long filopodia (>10 μm in length) requires the rapid transport of key materials towards the growing end [14] and this process is facilitated by the myosin motor proteins such as myosin-X or myosin V using an ATP-dependent ‘walking’ mechanism. Although the extension of filopodia is often described in a highly ordered manner, and does rely on the defined movements of Myosin-X for component delivery to the filopodia tip, the contribution of random diffusion of components must also be considered. Stochastic simulation models were recently presented that describe such phenomena, where ‘molecular noise’ may influence concentration gradients of G-actin as well as efficacy of the machinery responsible for filopodia growth.
One such study indicates that although the spatial distribution of static Myosin-X is universally consistent, and not altered by organelle length, the concentration of walking motors may vary. “Traffic jams” of myosin-X may occur for example at the base of the filopodia. Although logically this will impede progression of the proteins and their cargo down the filament, it was calculated that following a build up of G-actin at the blockage site, a concentration gradient is generated that enables its diffusion down the filopodia, (so long as the G-actin is not sequestered by the blocked motors) which subsequently sustains filopodia extension [15]. Similarly, the constant association and dissociation of capping protein at the barbed ends of actin filaments has been shown, also using stochastic simulations, to influence the dynamics of filopodia extension. In this case amplification of these regulatory proteins from initially low concentrations may trigger the fast retrograde flow of actin and induce repeated extension-retraction cycles that occur on a micro-meter scale. Compared to actin-only models, these dynamic cycles enable the growth of substantially longer filopodia [16].
References
- Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, and Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 2003; 160(3):409-21. [PMID: 12566431]
- Tokuo H, and Ikebe M. Myosin X transports Mena/VASP to the tip of filopodia. Biochem. Biophys. Res. Commun. 2004; 319(1):214-20. [PMID: 15158464]
- Schirenbeck A, Arasada R, Bretschneider T, Stradal TEB, Schleicher M, and Faix J. The bundling activity of vasodilator-stimulated phosphoprotein is required for filopodium formation. Proc. Natl. Acad. Sci. U.S.A. 2006; 103(20):7694-9. [PMID: 16675552]
- Lebrand C, Dent EW, Strasser GA, Lanier LM, Krause M, Svitkina TM, Borisy GG, and Gertler FB. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron 2004; 42(1):37-49. [PMID: 15066263]
- Han Y, Chung CY, Wessels D, Stephens S, Titus MA, Soll DR, and Firtel RA. Requirement of a vasodilator-stimulated phosphoprotein family member for cell adhesion, the formation of filopodia, and chemotaxis in dictyostelium. J. Biol. Chem. 2002; 277(51):49877-87. [PMID: 12388544]
- Mogilner A, and Rubinstein B. The physics of filopodial protrusion. Biophys. J. 2005; 89(2):782-95. [PMID: 15879474]
- Svitkina TM, and Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 1999; 145(5):1009-26. [PMID: 10352018]
- Small JV. Lamellipodia architecture: actin filament turnover and the lateral flow of actin filaments during motility. Semin. Cell Biol. 1994; 5(3):157-63. [PMID: 7919229]
- Mogilner A, and Oster G. Cell motility driven by actin polymerization. Biophys. J. 1996; 71(6):3030-45. [PMID: 8968574]
- Lewis AK, and Bridgman PC. Nerve growth cone lamellipodia contain two populations of actin filaments that differ in organization and polarity. J. Cell Biol. 1992; 119(5):1219-43. [PMID: 1447299]
- Tseng Y, Kole TP, Lee JSH, Fedorov E, Almo SC, Schafer BW, and Wirtz D. How actin crosslinking and bundling proteins cooperate to generate an enhanced cell mechanical response. Biochem. Biophys. Res. Commun. 2005; 334(1):183-92. [PMID: 15992772]
- Coleman TR, and Mooseker MS. Effects of actin filament cross-linking and filament length on actin-myosin interaction. J. Cell Biol. 1985; 101(5 Pt 1):1850-7. [PMID: 2932451]
- Watanabe TM, Tokuo H, Gonda K, Higuchi H, and Ikebe M. Myosin-X induces filopodia by multiple elongation mechanism. J. Biol. Chem. 2010; 285(25):19605-14. [PMID: 20392702]
- Bohil AB, Robertson BW, and Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc. Natl. Acad. Sci. U.S.A. 2006; 103(33):12411-6. [PMID: 16894163]
- Zhang H, Berg JS, Li Z, Wang Y, Lång P, Sousa AD, Bhaskar A, Cheney RE, and Strömblad S. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat. Cell Biol. 2004; 6(6):523-31. [PMID: 15156152]
- Schmidt CE, Dai J, Lauffenburger DA, Sheetz MP, and Horwitz AF. Integrin-cytoskeletal interactions in neuronal growth cones. J. Neurosci. 1995; 15(5 Pt 1):3400-7. [PMID: 7751919]
- Zhuravlev PI, Lan Y, Minakova MS, and Papoian GA. Theory of active transport in filopodia and stereocilia. Proc. Natl. Acad. Sci. U.S.A. 2012; 109(27):10849-54. [PMID: 22711803]
- Zhuravlev PI, and Papoian GA. Molecular noise of capping protein binding induces macroscopic instability in filopodial dynamics. Proc. Natl. Acad. Sci. U.S.A. 2009; 106(28):11570-5. [PMID: 19556544]


