How do post-translational modifications of cell-cell adhesions impact their stability?
Post-translational modification of adhesion complex components is one of the most complicated aspects in the regulation of cell-cell adhesions. It involves an array of protein enzymes and despite a growing body of knowledge on the topic, many of the specific mechanisms, from recruitment of regulators, to the consequence each modification has on adhesion stability and function has yet to be described.
Phosphorylation events are the most prominent posttranslational modification to play a role in the regulation of cell-cell adhesions. These are mediated by phosphatases and kinases, of which at least 34 are known to localize to AJs alone [1]. In many cases the method by which phosphatases/kinases are recruited to the adhesion site has yet to be established, however, in most cases direct binding with either core components or scaffolding proteins is likely to be involved. Indeed this has been established in some specific cases; for example CSK and VE-PTP bind directly to VE-cadherin [2], MET and PTPRF to β-catenin [3][4] and ROCK1 to p120-catenin [12].
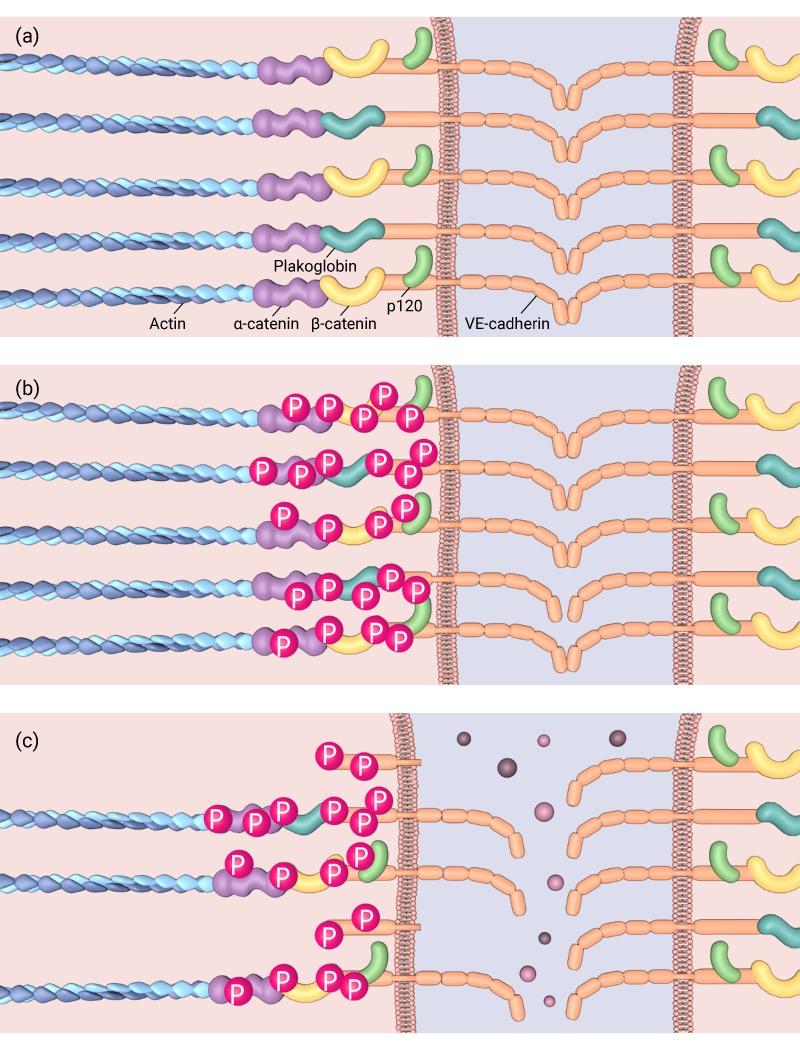
Phosphorylation of cell-cell adhesion components can affect the adhesions’ stabilisation, (a) Normal adherens junction, (b) Tyrosine phosphorylation of VE-cadherin is induced, which can lead to (c) cleavage of VE-cadherin and weakening of the adherens junction
Addition or removal of a phosphate molecule may not only alter the targets binding affinity to other components, but may activate or deactivate enzymatic properties to produce either positive (stabilizing), or negative (destabilizing) consequences. Furthermore, the activity of kinases/phosphatases is often correlated to the action of the cell-cell adhesion complex, with mechanical cues from neighboring cells triggering the enzymatic activity of the protein. This was observed, for example, when the H-Ras/Raf/MEK/ERK signaling cascade was induced following the attachment of invasive breast cancer cells to endothelial cells in vitro. Here, tyrosine phosphorylation of VE-cadherin was induced, and subsequently, the dissociation of β-catenin from the complex observed [5].
Similarly, attachment of neutrophils and lymphocytes to endothelial cells activated by TNF-α lead to the rapid dissociation of the tyrosine phosphatase VE-PTP from VE-cadherin [2]. VE-PTP had previously been found to bind to, and reduce the phosphorylation of VE-cadherin, but not β-catenin [6], which effectively stabilized the adhesion complex. Leukocyte mediated dissociation of VE-PTP from VE-cadherin was associated with increased phosphorylation of VE-cadherin as well as β-catenin and plakoglobin. Importantly, it was the plakoglobin that was found to be essential in VE-PTP stabilization VE-cadherin [2].
As mentioned, leukocyte mediated dissociation of VE-PTP was only observed in cells that had been activated by the inflammatory cytokine, TNF-α, highlighting that regulators themselves may be activated only under specific cellular conditions. This was again reflected when the tyrosine phosphatase SHP2 was found to dissociate from β-catenin as a result of thrombin stimulation [7]. Later studies noted that dissociation of SHP2 promoted catenin phosphorylation [8], confirming it to be a substrate of SHP2 [9], and revealed, through silencing or inhibition of SHP2 its role in promoting the mobility of E-cadherin, and hence, the integrity of adhesion complexes [21]).
Similarly, posttranslational modification of scaffolding proteins such as p120-catenin may be equally influential to the stability of the adhesion complex. This was demonstrated when it was found that PKCa mediated phosphorylation of p120-catenin at Serine 879 which initiated dissociation of p120-catenin from the AJ, and subsequently promoted its disassembly [10].
References
- Bertocchi C, Vaman Rao M, and Zaidel-Bar R. Regulation of adherens junction dynamics by phosphorylation switches. J Signal Transduct 2012; 2012:125295. [PMID: 22848810]
- Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, Bixel MG, Butz S, and Vestweber D. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J. Exp. Med. 2008; 205(12):2929-45. [PMID: 19015309]
- Monga SPS, Mars WM, Pediaditakis P, Bell A, Mulé K, Bowen WC, Wang X, Zarnegar R, and Michalopoulos GK. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002; 62(7):2064-71. [PMID: 11929826]
- Aicher B, Lerch MM, Müller T, Schilling J, and Ullrich A. Cellular redistribution of protein tyrosine phosphatases LAR and PTPsigma by inducible proteolytic processing. J. Cell Biol. 1997; 138(3):681-96. [PMID: 9245795]
- Dejana E, Orsenigo F, and Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell. Sci. 2008; 121(Pt 13):2115-22. [PMID: 18565824]
- Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, and Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002; 21(18):4885-95. [PMID: 12234928]
- Ukropec JA, Hollinger MK, Salva SM, and Woolkalis MJ. SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J. Biol. Chem. 2000; 275(8):5983-6. [PMID: 10681592]
- Lee S, Won J, Lee H, Lee H, Youn S, Lee J, Cho C, Cho H, Oh S, Chae I, and Kim H. Angiopoietin-1 protects heart against ischemia/reperfusion injury through VE-cadherin dephosphorylation and myocardiac integrin-β1/ERK/caspase-9 phosphorylation cascade. Mol. Med. 2011; 17(9-10):1095-106. [PMID: 21738954]
- Timmerman I, Hoogenboezem M, Bennett AM, Geerts D, Hordijk PL, and van Buul JD. The tyrosine phosphatase SHP2 regulates recovery of endothelial adherens junctions through control of β-catenin phosphorylation. Mol. Biol. Cell 2012; 23(21):4212-25. [PMID: 22956765]
- Vandenbroucke St Amant E, Tauseef M, Vogel SM, Gao X, Mehta D, Komarova YA, and Malik AB. PKCα activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity. Circ. Res. 2012; 111(6):739-49. [PMID: 22798526]


