How do nascent adhesions grow into mature focal adhesions?
Nascent adhesions undergo a transient phase of rapid assembly and disassembly through which a fraction of adhesions survive to evolve into more stable and larger focal adhesions. Typically adhesions during this time vary in size between 0.5 to 1µm, with an average lifetime of ~80 s [1]. They are largely found at the boundary between the lamellum and lamellipodial ruffles [2][3]. These structures help strengthen the physical linkage between the extracellular matrix (ECM) and the loosely packed actin network. Moreover, they transmit mechanical and chemical signals via mechanosensitive components that can act as both scaffolds and signaling molecules.
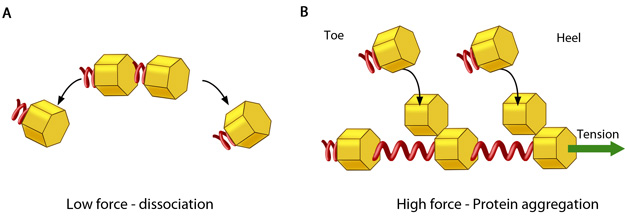
In the absence of or under low actomyosin contractile forces, adhesion components start dispersing. In such scenarios, the unidirectional shear force (not shown) from the actin retrograde flow may also aid dissociation. B. Under substantial pulling ‘tension’ from actomyosin contractions, aggregation of adhesion components occurs in the direction of pulling. The protein complexes of adhesions (cell’s feet) can be considered as elastic units that expand under force to accommodate new components towards one end (heel). The other end of the adhesion structure that faces the actin retrograde movement is termed ‘toe’. Adapted from [18].
- The retrograde flow of actin in lamellipodial ruffles [7][8]
- The remodeling of the dendritic lamellipodial actin networks into linear filaments array of the stress fibers in the vicinity of adhesions at the lamellipodium-lamellum interface [7][9][10][11]
- The bundling of actin by myosin II, which distally interacts with adhesions ([1], reviewed in [12]) and stimulates proximal actin assembly [13].
Actomyosin contractility also plays crucial role at later stages.
Since these forces are funneled through mechanosensitive elements of the adhesions, this is believed to result in conformational changes in the actin linking components that subsequently promote the continued assembly of components at sites of adhesion [14][15] (reviewed in [16], see figure below). Conversely, the reduction of tension leads to adhesion dissociation. Adhesion reinforcement can be therefore be thought of as force-induced, anisotropic protein aggregation along the direction of the force [5][17]. This ultimately leads to increased cellular stiffness. However these later stages of adhesion growth are thought to be independent of substrate stiffness [4].
Adhesion reinforcement has also been explained as a change in the chemical potential of protein aggregates that favors self assembly [18]. For example; rapid binding or breaking of weak β3 integrin-ECM ligand bonds enable continuous force-sensing, resulting in the recruitment of ]Src to integrin tails [19][20] and the stimulation of FAK-mediated phosphorylation in a tension-dependent manner that ultimately promotes vinculin recruitment [21].
The organization of actin into filaments near adhesion sites (reviewed in [12]) and the inward translocation of α5β1 integrins [22] serves as a physical template for further elongation. These spatial constraints orient growing adhesions in a centripetal fashion[23][24]. α-actinin is one of the key orchestrators of elongation, likely setting up the template along which actin filaments will extend and adhesions will grow [25]. Adhesion elongation is also regulated by continual Rac activation [26].
References
- Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, and Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 2008; 10(9):1039-50. [PMID: 19160484]
- Bershadsky AD, Tint IS, Neyfakh AA, and Vasiliev JM. Focal contacts of normal and RSV-transformed quail cells. Hypothesis of the transformation-induced deficient maturation of focal contacts. Exp. Cell Res. 1985; 158(2):433-44. [PMID: 2988988]
- Nobes CD, and Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995; 81(1):53-62. [PMID: 7536630]
- Walcott S, Kim D, Wirtz D, and Sun SX. Nucleation and decay initiation are the stiffness-sensitive phases of focal adhesion maturation. Biophys. J. 2011; 101(12):2919-28. [PMID: 22208190]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, and Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001; 3(5):466-72. [PMID: 11331874]
- Beningo KA, Dembo M, Kaverina I, Small JV, and Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 2001; 153(4):881-8. [PMID: 11352946]
- Hotulainen P, and Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 2006; 173(3):383-94. [PMID: 16651381]
- Alexandrova AY, Arnold K, Schaub S, Vasiliev JM, Meister J, Bershadsky AD, and Verkhovsky AB. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE 2008; 3(9):e3234. [PMID: 18800171]
- Endlich N, Otey CA, Kriz W, and Endlich K. Movement of stress fibers away from focal adhesions identifies focal adhesions as sites of stress fiber assembly in stationary cells. Cell Motil. Cytoskeleton 2007; 64(12):966-76. [PMID: 17868136]
- Shemesh T, Verkhovsky AB, Svitkina TM, Bershadsky AD, and Kozlov MM. Role of focal adhesions and mechanical stresses in the formation and progression of the lamellipodium-lamellum interface [corrected]. Biophys. J. 2009; 97(5):1254-64. [PMID: 19720013]
- Walcott S, and Sun SX. A mechanical model of actin stress fiber formation and substrate elasticity sensing in adherent cells. Proc. Natl. Acad. Sci. U.S.A. 2010; 107(17):7757-62. [PMID: 20385838]
- Vicente-Manzanares M, Ma X, Adelstein RS, and Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009; 10(11):778-90. [PMID: 19851336]
- Rossier OM, Gauthier N, Biais N, Vonnegut W, Fardin M, Avigan P, Heller ER, Mathur A, Ghassemi S, Koeckert MS, Hone JC, and Sheetz MP. Force generated by actomyosin contraction builds bridges between adhesive contacts. EMBO J. 2010; 29(6):1055-68. [PMID: 20150894]
- Zamir E, and Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J. Cell. Sci. 2001; 114(Pt 20):3583-90. [PMID: 11707510]
- Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Döbereiner H, Freund Y, Borisy G, and Sheetz MP. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell 2007; 128(3):561-75. [PMID: 17289574]
- Geiger B, Spatz JP, and Bershadsky AD. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009; 10(1):21-33. [PMID: 19197329]
- Besser A, and Safran SA. Force-induced adsorption and anisotropic growth of focal adhesions. Biophys. J. 2006; 90(10):3469-84. [PMID: 16513789]
- Shemesh T, Geiger B, Bershadsky AD, and Kozlov MM. Focal adhesions as mechanosensors: a physical mechanism. Proc. Natl. Acad. Sci. U.S.A. 2005; 102(35):12383-8. [PMID: 16113084]
- Felsenfeld DP, Schwartzberg PL, Venegas A, Tse R, and Sheetz MP. Selective regulation of integrin–cytoskeleton interactions by the tyrosine kinase Src. Nat. Cell Biol. 1999; 1(4):200-6. [PMID: 10559917]
- Roca-Cusachs P, Gauthier NC, Del Rio A, and Sheetz MP. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc. Natl. Acad. Sci. U.S.A. 2009; 106(38):16245-50. [PMID: 19805288]
- Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, and Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol. 2010; 188(6):877-90. [PMID: 20308429]
- Huveneers S, Truong H, Fässler R, Sonnenberg A, and Danen EHJ. Binding of soluble fibronectin to integrin alpha5 beta1 – link to focal adhesion redistribution and contractile shape. J. Cell. Sci. 2008; 121(Pt 15):2452-62. [PMID: 18611961]
- Zaidel-Bar R, Ballestrem C, Kam Z, and Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell. Sci. 2003; 116(Pt 22):4605-13. [PMID: 14576354]
- Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, and Kozlov MM. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur. J. Cell Biol. 2005; 85(3-4):165-73. [PMID: 16360240]
- Triplett JW, and Pavalko FM. Disruption of alpha-actinin-integrin interactions at focal adhesions renders osteoblasts susceptible to apoptosis. Am. J. Physiol., Cell Physiol. 2006; 291(5):C909-21. [PMID: 16807302]
- Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, and Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J. Cell Biol. 2006; 173(4):587-9. [PMID: 16717130]


