How do invadopodia degrade the extracellular matrix?
The main function attributed to invadopodia is that of extracellular matrix (ECM) degradation, facilitated by the secretion of proteases. Maintenance of this process requires the delivery of new proteases from the Golgi, which is conveniently positioned in close proximity to invadopodia [1]. Indeed, the treatment of cells with Brefeldin A, an inhibitor of ER (endoplasmic reticulum) to Golgi transport, has been shown to prevent ECM degradation by invadopodia [2]. The Ena/VASP family protein, Mena, is also implicated in this process. More specifically the MenaINV isoform that favors cancer cell invasiveness by promoting the stabilization of invadopodia and enhancing their ECM-degrading activity [3][4].
ECM degradation itself is carried out by a variety of secreted matrix metalloproteinases (MMPs) and serine proteases. Currently over 25 different MMPs have been identified that together have the potential to degrade the entire ECM [5]. Of all the MMPs, MMP14 (also known as MT1-MMP), is considered to be the major regulator of invadopodia-mediated ECM degradation across several cell models [6]. MMP docking has been shown to be essential for ECM degradation in melanoma cells, with knock-down [7] or overexpression [5] showing a decrease or increase in invadopodial activity, respectively. Cortactin, commonly known as a weak activator of the actin nucleator Arp2/3, also has a role to play in MMP secretion and ECM degradation [6].
Cortactin accumulation has been shown to precede MMP accumulation at the tips of invadopodia and has been suggested to regulate their secretion [7]. This cortactin-dependent maturation process has been shown to be dependent on the activity of LIM kinases [8].
How do matrix metalloproteinases (MMPs) facilitate extracellular matrix disassembly?
Extracellular matrix disassembly is the degradation of the matrix components by proteases such as matrix metalloproteinases, and is a key step in normal physiological functions such as embryonic development and tissue remodeling, as well as in pathological conditions such as cancer progression.
Matrix metalloproteinases (MMPs)
Matrix metalloproteinases (MMPs) are a family of proteases that digest components of the extracellular matrix (ECM) and surface receptors. The first MMP was identified in the 1960s as an enzyme which could digest the ECM component collagen and so was named collagenase [9]. Following this initial discovery a whole family of structurally related proteases were discovered. These proteases were all able to digest ECM components and did so in a zinc dependent manner. This led to the renaming of this protein family to matrix metalloproteinases. There are currently 23 human MMPs and 24 mouse MMPs [10], in addition to MMP homologs in invertebrates such as the fruit fly Drosophila melanogaster [10].
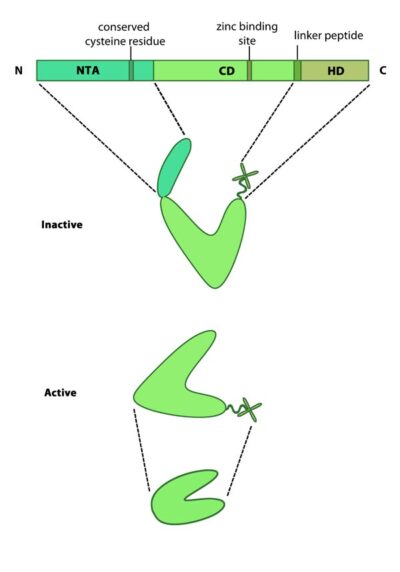
This schematic diagram illustrates the molecular organization of MMPs. Relevant domains believed to be important for MMP activity are highlighted.
Matrix metalloproteinase structure
Mammalian MMPs comprise three major domains; an N terminal auto-inhibitory pro-domain, a catalytic domain and a C terminal hemopexin domain (see Figure at top right) [11]. MMPs are produced as inactive precursors. Their activity is inhibited through a conserved cysteine residue residing within the N terminal auto-inhibitory pro-domain. This residue interacts with the zinc ion bound to the catalytic domain and prevents cleavage of the substrate. The catalytic domain is connected to the hemopexin domain via a flexible linker peptide. The hemopexin domain forms a beta propeller structure, made of four ‘blades’ each of which represent a single hemopexin module. This domain regulates protein-protein interactions, substrate specificity and enzyme activity via interactions with tissue inhibitors of matrix metalloproteinases (TIMPs) [12].
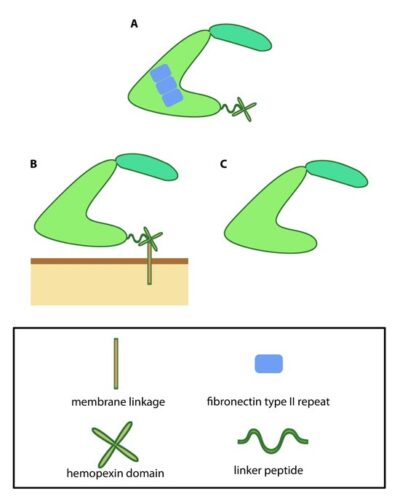
This schematic diagram illustrates variations on the common MMP structure (reviewed in [3]). (A) MMP2 and MMP9 have additional fibronectin domain inserts within the catalytic domain (B) Membrane bound MMPs (MT-MMPs) contain an additional membrane linkage domain attached to the hemopexin domain, in the form of either a GPI (glycosylphosphatidylinositol) anchor or a transmembrane domain. (C) Minimal MMPs, such as MMP7 and MMP26, do not contain a linker peptide or hemopexin domain.
Activation of matrix metalloproteinases
MMPs are produced as inactive precursors which are exposed to the extracellular milieu either via secretion or translocation to the membrane. Once exposed to the extracellular environment they are activated through proteolytic cleavage of the N terminal autoinhibitory domain.
Cleavage is carried out by a range of different proteases including the MMPs themselves, as well as tissue proteases such as tryptase, plasmin and kallikrein [12]. Following activation, MMPs are able to digest various ECM components including a host of collagen types (I-V, VII, X, XI), gelatin, laminin, basement membrane proteins entactin and perlecan and ECM glycoproteins such as tenascin [12]. Active MMPs are involved in a wide range of cellular processes including; tissue remodeling, cell survival, immune cell migration and cancer pathology [11].
References
- Baldassarre M, Pompeo A, Beznoussenko G, Castaldi C, Cortellino S, McNiven MA, Luini A, and Buccione R. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol. Biol. Cell 2003; 14(3):1074-84. [PMID: 12631724]
- Buccione R, Caldieri G, and Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009; 28(1-2):137-49. [PMID: 19153671]
- Albiges-Rizo C, Destaing O, Fourcade B, Planus E, and Block MR. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J. Cell. Sci. 2009; 122(Pt 17):3037-49. [PMID: 19692590]
- Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim H, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, Sahai E, Condeelis JS, and Gertler FB. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev. Cell 2008; 15(6):813-28. [PMID: 19081071]
- Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, and Chen WT. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc. Natl. Acad. Sci. U.S.A. 1997; 94(15):7959-64. [PMID: 9223295]
- Clark ES, Whigham AS, Yarbrough WG, and Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007; 67(9):4227-35. [PMID: 17483334]
- Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, and Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006; 66(6):3034-43. [PMID: 16540652]
- Scott RW, Hooper S, Crighton D, Li A, König I, Munro J, Trivier E, Wickman G, Morin P, Croft DR, Dawson J, Machesky L, Anderson KI, Sahai EA, and Olson MF. LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. J. Cell Biol. 2010; 191(1):169-85. [PMID: 20876278]
- GROSS J, and LAPIERE CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc. Natl. Acad. Sci. U.S.A. 1962; 48:1014-22. [PMID: 13902219]
- Page-McCaw A. Remodeling the model organism: matrix metalloproteinase functions in invertebrates. Semin. Cell Dev. Biol. 2007; 19(1):14-23. [PMID: 17702617]
- Page-McCaw A, Ewald AJ, and Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007; 8(3):221-33. [PMID: 17318226]
- Overall CM. Molecular determinants of metalloproteinase substrate specificity: matrix metalloproteinase substrate binding domains, modules, and exosites. Mol. Biotechnol. 2002; 22(1):51-86. [PMID: 12353914]


