How do Focal Adhesions Facilitate Mechanosensing?
Focal adhesions are known to sense both chemical and physical properties of their matrix environment. Chemical sensing is mediated by the different types of receptors that may function additively, synergistically or antagonistically [1],[2],[3],[4]. However, even with the molecular repertoire of focal adhesions (FAs) having been unveiled substantially (reviewed in [5], [6]), the details of functional interplay between these proteins on sensing mechanical forces remain fairly elusive. From available data, it can only be suggested that FAs undergo regulated turnover at any given time governed by diverse environmental signals (reviewed in[7]).
Matrix density, spacing [8],[9], rigidity [10], orientation and geometry [11] are some of the physical parameters the focal adhesions and in turn, cells are known to be sensitive to. However, over years, the mechanisms that integrate such complex information have not been understood. Only recently, the key determinants of rigidity sensing by integrins have been described (reviewed in [12],[13]):
- The strength of integrin-ECM bonds
- Rates of cell protrusion/ retraction
- Force produced during protrusion/ retraction and
- Elasticity of associated mechanosensitive proteins
A remarkable feature of cell-matrix adhesions, which makes them more versatile than any individual surface receptor, is the large repertoire of mechanosensitive and signaling components in their cytoplasmic scaffold. Mechanotransduction most likely occurs through protein conformational changes in multi-modular proteins, that act as molecular switches [13] (reviewed in [14]) leading to subsequent phosphorylation signaling pathways or addition of components [15] (reviewed in [16]) under internal/external mechanical perturbation [17]. These ultimately affect prominent protein-protein interactions allowing self-assembly and/or remodeling of the adhesion unit [18]. These allow a multitude of possibilities that trigger force-induced signal transduction pathways involving the adhesion components and diverse targets, resulting in cascading events at a distance (reviewed in [14]).
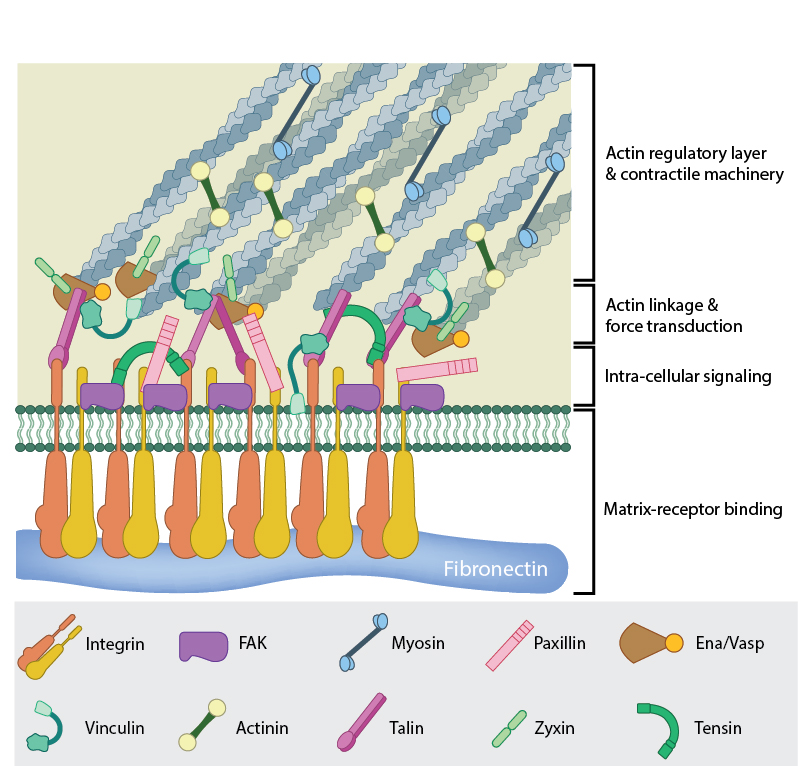
A mature FA contains hundreds of proteins that are grouped based on their contribution to four basic processes: receptor/matrix binding, linkage to actin cytoskeleton, intracellular signal transduction, and actin polymerization. Both actin polymerization and actomyosin contractile machinery generate forces that affect mechanosensitive proteins in the actin linking module, the receptor module (e.g. integrins), the signaling module, and the actin polymerization module. The combined activity of the mechanosensitive components form the mechanoresponsive network. The theoretical organization and protein-protein interactions as shown are based upon references.
References
- Heino J, and Käpylä J. Cellular receptors of extracellular matrix molecules. Curr. Pharm. Des. 2009; 15(12):1309-17. [PMID: 19355970]
- Morgan MR, Humphries MJ, and Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 2007; 8(12):957-69. [PMID: 17971838]
- Bass MD, Morgan MR, and Humphries MJ. Syndecans shed their reputation as inert molecules. Sci Signal 2009; 2(64):pe18. [PMID: 19336838]
- Wei Y, Yang X, Liu Q, Wilkins JA, and Chapman HA. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 1999; 144(6):1285-94. [PMID: 10087270]
- Zamir E, and Geiger B. Components of cell-matrix adhesions. J. Cell. Sci. 2001; 114(Pt 20):3577-9. [PMID: 11707509]
- Lo SH. Focal adhesions: what’s new inside. Dev. Biol. 2006; 294(2):280-91. [PMID: 16650401]
- Bershadsky AD, Balaban NQ, and Geiger B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003; 19:677-95. [PMID: 14570586]
- Cavalcanti-Adam EA, Volberg T, Micoulet A, Kessler H, Geiger B, and Spatz JP. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 2007; 92(8):2964-74. [PMID: 17277192]
- Schvartzman M, Palma M, Sable J, Abramson J, Hu X, Sheetz MP, and Wind SJ. Nanolithographic control of the spatial organization of cellular adhesion receptors at the single-molecule level. Nano Lett. 2011; 11(3):1306-12. [PMID: 21319842]
- Prager-Khoutorsky M, Lichtenstein A, Krishnan R, Rajendran K, Mayo A, Kam Z, Geiger B, and Bershadsky AD. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol. 2011; 13(12):1457-65. [PMID: 22081092]
- Vogel V, and Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006; 7(4):265-75. [PMID: 16607289]
- Gardel ML, Schneider IC, Aratyn-Schaus Y, and Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu. Rev. Cell Dev. Biol. 2010; 26:315-33. [PMID: 19575647]
- Moore SW, Roca-Cusachs P, and Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev. Cell 2010; 19(2):194-206. [PMID: 20708583]
- Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct 2006; 35:459-88. [PMID: 16689645]
- Ballestrem C, Erez N, Kirchner J, Kam Z, Bershadsky A, and Geiger B. Molecular mapping of tyrosine-phosphorylated proteins in focal adhesions using fluorescence resonance energy transfer. J. Cell. Sci. 2006; 119(Pt 5):866-75. [PMID: 16478788]
- Giannone G, and Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006; 16(4):213-23. [PMID: 16529933]
- Johnson CP, Tang H, Carag C, Speicher DW, and Discher DE. Forced unfolding of proteins within cells. Science 2007; 317(5838):663-6. [PMID: 17673662]
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, and Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 2001; 153(6):1175-86. [PMID: 11402062]


