How do focal adhesions act as molecular clutches in lamellipodia?
Once formed, focal adhesions essentially act as “molecular clutches”, promoting protrusion at the leading edge whilst suppressing membrane contraction (reviewed in [1] [2][3]). Adhesions aid forward movement by regulating the forces produced by actin dynamics in different cellular compartments through several methods:
1) They aid membrane protrusion by resisting actin retrograde flow [4] and hence, indirectly promote the force produced by lamellipodial actin polymerization.
2) They convert myosin pulling forces at the lamellar interface into traction forces against the ECM that pulls the cell body forward [5][6].
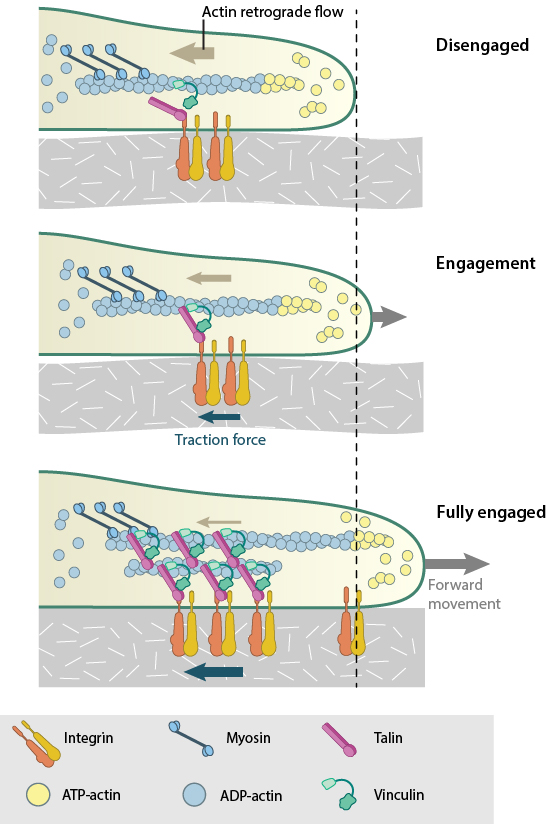
Actin filaments are constantly undergoing polymerization and depolymerization, resulting in retrograde flow. The ‘molecular clutch’ engages when actin filaments connect to integrin to form focal adhesions. The resistance provided by the focal adhesions against retrograde flow promotes membrane protrusion. Also, contractile forces generated by myosin are converted by the focal adhesion into traction forces against that ECM. Combined, these forces drive forward movement by membrane protrusion. As the focal adhesions mature, the connection to actin is reinforced and the clutch become fully engaged.
These two methods are interdependent and cooperatively contribute to the propulsive forces generated at the leading edge. The efficiency of the molecular clutch in converting this force into protrusion is variable. Because some components of the adhesion complex move along with the retrograde flow [7], the clutch slips [8]. The rate of forward protrusion increases however when actin and actin-adhesion linking components become more organized [9] and the retrograde flow of actin is subsequently slowed down [4][10]. In this case the clutch can be described as “partially engaged”. As the adhesions grow and mature under stress, the clutch transforms from being “partially/locally engaged” to “engaged” [1] and hence can influence global cell behavior [11][12].
How is actin flow related to traction stress during lamellipodia extension?
Experiments have demonstrated that a biphasic relationship exists between the rate of actin flow and traction stress [13]. Whilst they are inversely related in the lamellipodium where nascent adhesions are formed and actin flow rate is high, the relationship becomes linear in areas with larger adhesions and slow actin flow [4], generating maximal propulsion at intermediate flow rates [14].
Two recent studies propose stochastic models that explain the state of adhesion clutches in these two regimes [15][16] (reviewed in [17]). One study describes how adhesion stability is modulated by the competition between energy required for maintaining the elastic bonds at the moving actin-clutch interface, and the energy dissipation that occurs when myosin pulls in the viscoelastic actin interior [15]. When the transmitted force and the speed of actin flow are moderate, much of the energy is invested in producing traction on the substrate. At this critical transition range, either an increase or decrease in the ratio of bound adhesion complexes could theoretically occur. In the second study however, based on known experimental results, it was assumed that at moderate actin flow rates adhesion clutches reach a quasi-equilibrium state between bound and unbound forms [16]. Thus the catch bond model of receptor-actin interactions are more convincing than the slip bond since it is more likely to lead to clutch engagement and adhesion growth, as observed in the experimental studies.
References
- Giannone G, Mège R, and Thoumine O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol. 2009; 19(9):475-86. [PMID: 19716305]
- Parsons JT, Horwitz AR, and Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010; 11(9):633-43. [PMID: 20729930]
- Gardel ML, Schneider IC, Aratyn-Schaus Y, and Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu. Rev. Cell Dev. Biol. 2010; 26:315-33. [PMID: 19575647]
- Alexandrova AY, Arnold K, Schaub S, Vasiliev JM, Meister J, Bershadsky AD, and Verkhovsky AB. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE 2008; 3(9):e3234. [PMID: 18800171]
- Suter DM, and Forscher P. Substrate-cytoskeletal coupling as a mechanism for the regulation of growth cone motility and guidance. J. Neurobiol. 2000; 44(2):97-113. [PMID: 10934315]
- Jurado C, Haserick JR, and Lee J. Slipping or gripping? Fluorescent speckle microscopy in fish keratocytes reveals two different mechanisms for generating a retrograde flow of actin. Mol. Biol. Cell 2004; 16(2):507-18. [PMID: 15548591]
- Guo W, and Wang Y. Retrograde fluxes of focal adhesion proteins in response to cell migration and mechanical signals. Mol. Biol. Cell 2007; 18(11):4519-27. [PMID: 17804814]
- Jiang G, Giannone G, Critchley DR, Fukumoto E, and Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 2003; 424(6946):334-7. [PMID: 12867986]
- Brown CM, Hebert B, Kolin DL, Zareno J, Whitmore L, Horwitz AR, and Wiseman PW. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J. Cell. Sci. 2006; 119(Pt 24):5204-14. [PMID: 17158922]
- Hu K, Ji L, Applegate KT, Danuser G, and Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science 2007; 315(5808):111-5. [PMID: 17204653]
- Assoian RK, and Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008; 18(7):347-52. [PMID: 18514521]
- Reddig PJ, and Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005; 24(3):425-39. [PMID: 16258730]
- Gardel ML, Sabass B, Ji L, Danuser G, Schwarz US, and Waterman CM. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J. Cell Biol. 2008; 183(6):999-1005. [PMID: 19075110]
- Beningo KA, Dembo M, Kaverina I, Small JV, and Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 2001; 153(4):881-8. [PMID: 11352946]
- Sabass B, and Schwarz US. Modeling cytoskeletal flow over adhesion sites: competition between stochastic bond dynamics and intracellular relaxation. J Phys Condens Matter 2010; 22(19):194112. [PMID: 21386438]
- Li Y, Bhimalapuram P, and Dinner AR. Model for how retrograde actin flow regulates adhesion traction stresses. J Phys Condens Matter 2010; 22(19):194113. [PMID: 21386439]
- Schwarz US, and Gardel ML. United we stand: integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction. J. Cell. Sci. 2012; 125(Pt 13):3051-60. [PMID: 22797913]


