How do filopodia pull on a substrate?
Although a reterograde motion of actin filaments is intrinsic in the formation of filopodia, the forces generated by actin treadmilling are too weak to facilitate the “pulling” mechanism required for rigidity sensing and other mechanosensing processes. This characteristic of filopodia is instead produced by the activity of myosin motor proteins such as Myosin II [1]. This process, as described in the Functional Module: ‘Microfilament Motor Activity’, is similar to that observed in the actin filament networks of the lamellipodia and lamella. Disassembly of actin bundles at the filopodial tip combined with the tension of the lipid bilayer also theoretically support the forces needed for pulling [2].
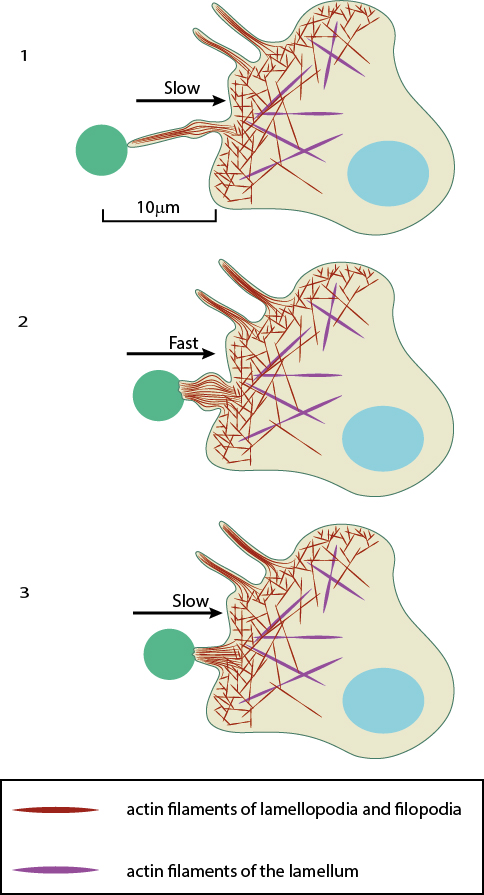
After a filopodium binds to an object, retrograde actin movement and myosin motor activity provide the force(s) needed for pulling the object towards the cell body. Once pulling starts, the initial slow movement (#1) is followed by a burst of rapid movement (#2) that diminishes as the object reaches the cell body (#3) [2].
In each case the mechanism behind pulling remains the same. While the powerstroke of Myosin II essentially drives the movement of filaments, the pulling process occurs over three distinct stages, each defined by varying rates of retraction. These can be described using the process by which pathogens are pulled towards macrophage cell bodies as an example. Here, immediately following filopodial adhesion to a pathogen, a slow motion retraction of the filopodia commences. This is followed by a phase of rapid movement where the pathogen is pulled towards the cell. A final phase of slow retraction occurs, resulting in the pathogen being fixed to the cell surface. This final phase of retraction is also characterized by a thickening of the filopodial base which is necessary to build-up large retraction forces for internalization [2][4]. Filopodial attachment to a surface (in addition to the ligand) produces counteracting adhesion forces which influence the retraction speed. In general, the rearward movement or ‘step size’ of a retracting filopodia decreases as the filopodia encounters a greater counteracting force [1]. Importantly, the mechanism may also be hijacked by a pathogen to ensure an efficient infection. This has been described for the Murine Leukemia Virus, amongst others [2]. In this case the virus binds to surface receptors at the tips of the filopodia and essentially rides the retracting actin filaments to entry points in the cell where it is internalized.
References
- Nishizaka T, Shi Q, and Sheetz MP. Position-dependent linkages of fibronectin- integrin-cytoskeleton. Proc. Natl. Acad. Sci. U.S.A. 2000; 97(2):692-7. [PMID: 10639141]
- Lehmann MJ, Sherer NM, Marks CB, Pypaert M, and Mothes W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J. Cell Biol. 2005; 170(2):317-25. [PMID: 16027225]
- Onfelt B, Nedvetzki S, Benninger RKP, Purbhoo MA, Sowinski S, Hume AN, Seabra MC, Neil MAA, French PMW, and Davis DM. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J. Immunol. 2006; 177(12):8476-83. [PMID: 17142745]
- Nagafusa T, Hoshino H, Sakurai T, Terakawa S, and Nagano A. Mechanical fragmentation and transportation of calcium phosphate substrate by filopodia and lamellipodia in a mature osteoclast. Cell Biol. Int. 2007; 31(10):1150-9. [PMID: 17498977]
- Schmidt CE, Dai J, Lauffenburger DA, Sheetz MP, and Horwitz AF. Integrin-cytoskeletal interactions in neuronal growth cones. J. Neurosci. 1995; 15(5 Pt 1):3400-7. [PMID: 7751919]


