What causes filopodia retraction and collapse?
Binding of filopodia to certain ligands or substratum may hinder filament assembly, thereby leading to changes that promote retraction, collapse or growth cone turning [1][2]. For example, substrate contacts with a repulsive signal on one side of a filopodium causes growth cone turning, while contact across the entire filopodial tip circumference causes complete filopodium collapse [1]. Normal retrograde flow of material continues under both circumstances and collapse is independent of the maintenance of growth cone protrusive activity [3]. Resorption and collapse are related processes and likely involve the same core proteins.
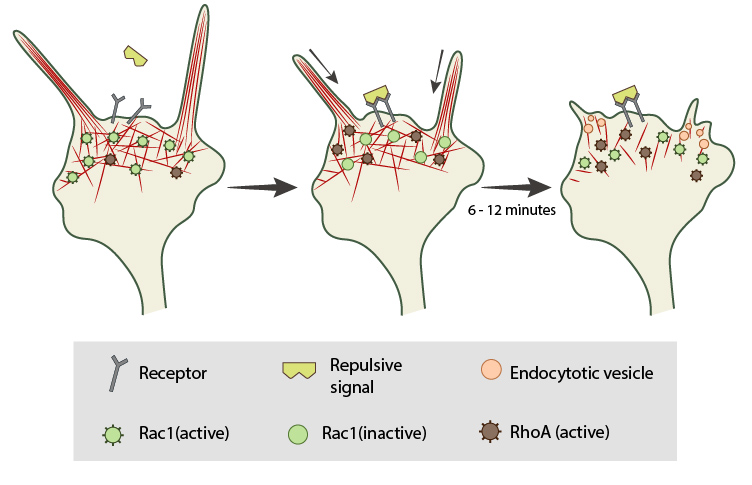
In neuronal growth cones, filopodia protrusion stops when a repulsive signal binds to its receptor on the cell surface. Receptor-binding transiently inactivates Rac1 GTPase and prevents it from promoting actin assembly. Resumption of Rac1 activity coincides with filopodia collapse and is required for endocytosis of the collapsing plasma membrane and reorganization of F-actin. RhoA and its effector, ROCK, are activated downstream of repulsive cues and their activity has been implicated in reducing actin polymerization following treatment with repulsive signals.
Rapid collapse produces a large number of filopodial strands tightly connected to the substrate by long tethers. F-actin bundles [4] and monomeric actin [2] disappear from collapsing filopodia without a compensatory rise in F-actin at the growth cone center; this indicates a net loss of actin rather than a rearward translocation. Furthermore, active nucleation and protrusion of filopodia is still found in discrete areas of collapsing growth cones, which argues against sequestration or modification of actin as the mechanism responsible for the loss of F-actin during the collapse [2]. It has also been shown that filopodial retraction involves periodic helical and rotational motion of the actin shaft, together with the retrograde flow of actin, and these dynamics were together responsible for filopodial shortening during retraction [5]
What regulates filopodia collapse and retraction?
A number of factors regulate collapse and retraction. For example, capping proteins promote filopodial retraction by shielding the barbed end of filaments from further assembly and elongation [6]. Inhibition of F-actin polymerization and protrusion during collapse are mediated by RhoA kinase activity [7]. Collapse may result from the exposure of a “naive” growth cone to a high concentration of a repellent followed by an overactive response [8]. The repulsive component appears to shut down the growth program and is therefore dominant over the growth-stimulating effects of adhesion molecules. In addition, the repellent also interferes with mechanisms that would normally result in filopodial retraction [1].
Why do filopodia move laterally?
Filopodia are motile structures, being able to extend, retract and move laterally as they sense their environment. Lateral movement is particularly important in allowing the structure to sense stimuli prior to its adhesion with another cell or substrate.
Lateral movement may be a consequence of filopodial protrusions being tilted with respect to the retrograde flow of actin [9][10]. This movement is inhibited once filopodia adhere to cells or substrates [11], however it is important to note that newly emerged filopodia seldom adhere to the substratum at their tips and are instead more likely to adhere at their bases [12].
Lateral movement and merging of filopodia may also stimulate further elongation of filopodia, suggesting that this may be an alternative method for promoting filopodial maturation and growth cone advancement on less adherent substrates [11].
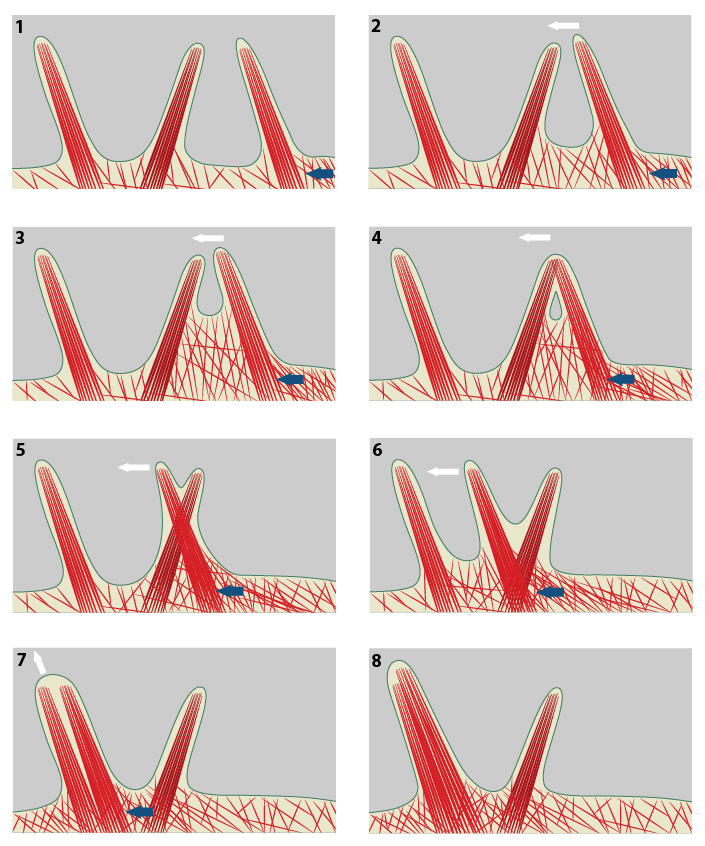
The direction of cell movement in a migrating cell is primarily controlled by actin filament assembly at the leading edge. In the diagram above, increased actin polymerization at the side of the filopodium (blue arrow) pushes the membrane forward and to the left (panel #1). The forces produced by actin polymerization against the actin bundles of a filopodium can lead to lateral movement (white arrow, panels #2 & 3). Under the microscope, filopodia appear to cross-over one another as distinct units when they are separated within the three-dimensional framework of the cell (panels #4-6). When a laterally moving filopodium encounters another filopodium within the same three-dimensional space, the two filopodium can fuse to become one; this frequently increases the width and length of the resulting filopodium (panels #7 & 8).
What causes filopodia stasis?
Typically filopodia are quite dynamic and are constantly growing or retracting. Thus, periods of stasis are often short-lived and even adhesion of the filopodial tip to a substrate will not last long before the cell pulls on the site and recruits additional components or retracts, leaving behind a thin tube of membrane. There are several events that may promote stasis in retracting filopodia.
1) Ligand binding to filopodial receptors and subsequent adhesion of the ligand/receptor complex to actin bundles:
After a ligand binds to a receptor on the filopodium, lateral connections between the receptor and the actin bundle(s) prevent retrograde actin movement relative to the ligand. This inhibition converts the previous retrograde movement of the retracting filaments into tension on the bundles. This tension might influence polymerization and stability of actin filaments and/or myosin activity.
2) Ligand binding to filopodial receptors followed by uncapping of filament barbed ends:
Retracting filopodia become static when receptor binding leads to filament uncapping and rapid polymerization at the barbed end. The retraction force is converted to retrograde flux and the filament length remains constant.
3) Capping of unstable actin filaments:
If retraction occurs by barbed end disassembly, capping of unstable filaments by an activated receptor will block retraction and induce stasis [13].
References
- Bastmeyer M, and Stuermer CA. Behavior of fish retinal growth cones encountering chick caudal tectal membranes: a time-lapse study on growth cone collapse. J. Neurobiol. 1993; 24(1):37-50. [PMID: 8419523]
- Fan J, Mansfield SG, Redmond T, Gordon-Weeks PR, and Raper JA. The organization of F-actin and microtubules in growth cones exposed to a brain-derived collapsing factor. J. Cell Biol. 1993; 121(4):867-78. [PMID: 8491778]
- Jurney WM, Gallo G, Letourneau PC, and McLoon SC. Rac1-mediated endocytosis during ephrin-A2- and semaphorin 3A-induced growth cone collapse. J. Neurosci. 2002; 22(14):6019-28. [PMID: 12122063]
- Zhou FQ, and Cohan CS. Growth cone collapse through coincident loss of actin bundles and leading edge actin without actin depolymerization. J. Cell Biol. 2001; 153(5):1071-84. [PMID: 11381091]
- Leijnse N, Oddershede LB, and Bendix PM. Helical buckling of actin inside filopodia generates traction. Proc. Natl. Acad. Sci. U.S.A. 2014; 112(1):136-41. [PMID: 25535347]
- Lin CH, Espreafico EM, Mooseker MS, and Forscher P. Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron 1996; 16(4):769-82. [PMID: 8607995]
- Gallo G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J. Cell. Sci. 2006; 119(Pt 16):3413-23. [PMID: 16899819]
- Walter J, Allsopp TE, and Bonhoeffer F. A common denominator of growth cone guidance and collapse? Trends Neurosci. 1990; 13(11):447-52. [PMID: 1701577]
- Small JV. Lamellipodia architecture: actin filament turnover and the lateral flow of actin filaments during motility. Semin. Cell Biol. 1994; 5(3):157-63. [PMID: 7919229]
- Oldenbourg R, Katoh K, and Danuser G. Mechanism of lateral movement of filopodia and radial actin bundles across neuronal growth cones. Biophys. J. 2000; 78(3):1176-82. [PMID: 10692307]
- Steketee MB, and Tosney KW. Three functionally distinct adhesions in filopodia: shaft adhesions control lamellar extension. J. Neurosci. 2002; 22(18):8071-83. [PMID: 12223561]
- Steketee M, Balazovich K, and Tosney KW. Filopodial initiation and a novel filament-organizing center, the focal ring. Mol. Biol. Cell 2001; 12(8):2378-95. [PMID: 11514623]
- Mitchison T, and Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron 1988; 1(9):761-72. [PMID: 3078414]


