How do filopodia attach to the surrounding substrate?
A diverse array of cellular responses can result when a filopodium makes contact with a ligand or substrate. These responses are dependent on the coupling of membrane-bound proteins to the backward (retrograde) flow of actin that drives filopodia elongation and motility. This contributes to larger processes such as cell pulling, which is needed for cell migration in wound healing, neurite growth [1], filopodia collapse and momentary stasis. These responses are influenced by the strength of the adhesion and it is known that contact differences between substrates or cell types influence the number of protruding filopodia [2].
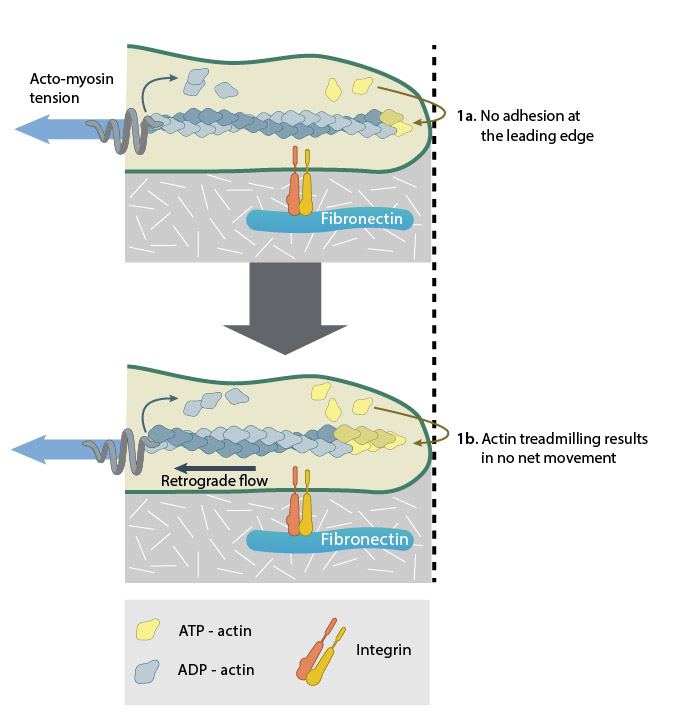
A lack of adhesion (1a) causes actin treadmilling to be converted mainly into retrograde flow (1b).
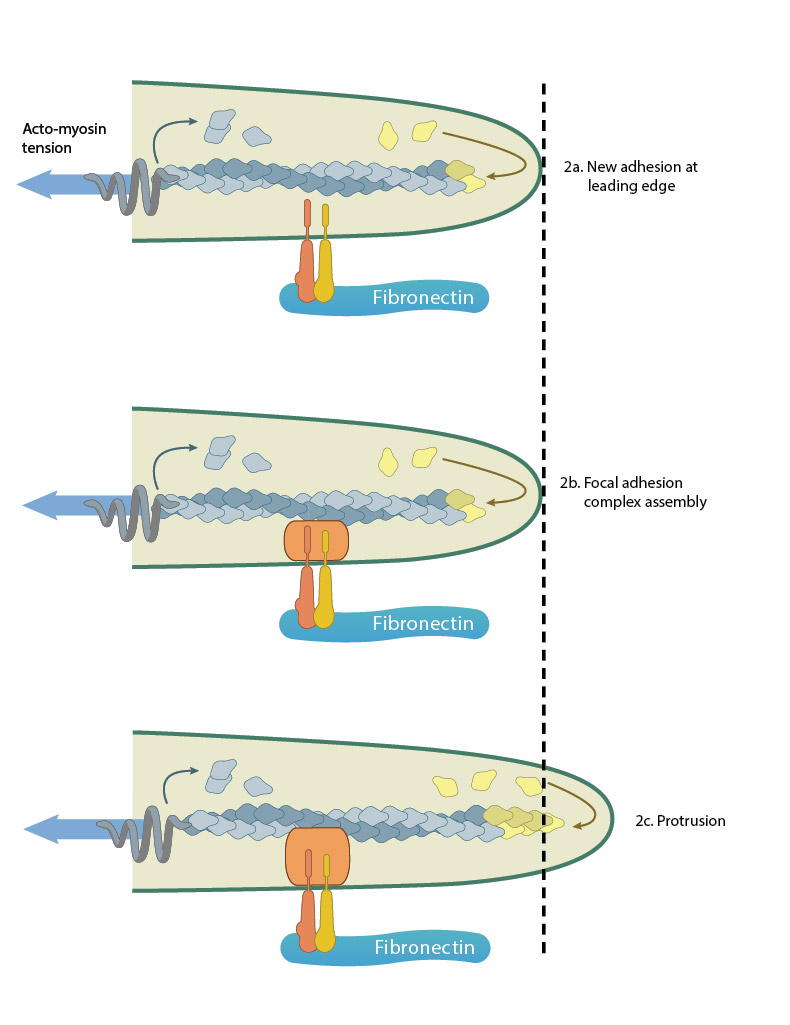
Adhesion acts like a “molecular grip” and influences the protrusion of filopodia (2a). Adhesion between membrane-anchored integrins and the extracellular substrate (e.g. fibronectin) causes clustering of focal adhesion components (represented by a hand,2b). Connections between the adhesions and the actin cytoskeleton converts the force generated by actin polymerization into protrusion(2c).
Three distinct types of adhesions can be identified within filopodia. Adhesion components may be localized to the tips of filopodia or be actively transported down the bundled actin filaments by myosin proteins. Each adhesion may function independently or work in concert to produce the overall guidance response:
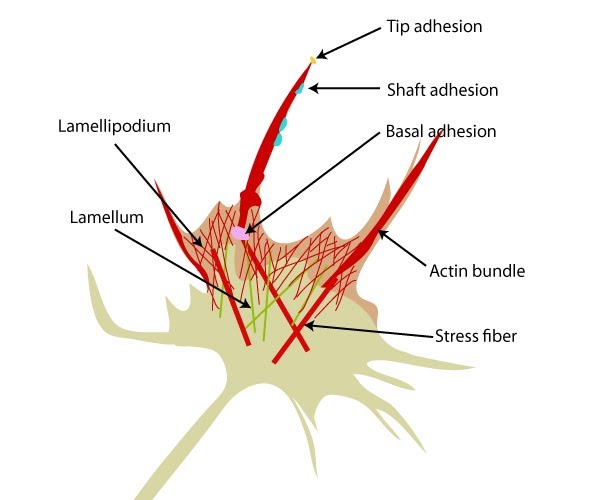
Three distinct adhesion types are found in filopodia: tip adhesions serve as guidance cues, shaft adhesions help control lamellipodial advance, and basal adhesions assist with filopodia emergence and are involved with components linking the cell to the substratum.
Tip adhesions
Filopodia on apposed cells interact directly through their tips [3] and/or ‘slide’ past each other (interdigitate) in order for adhesions to form (through cadherin-cadherin contact) between the tip of one filopodia and the cell membrane at the base of the adjacent filopodium [4]. Tip adhesions are likely to contain proteins that are found in nascent focal complexes [5][6][7]. Depending on the type of substrate, and strength of the adhesion, the resulting response may differ. In filopodia extending from neuronal growth cones, signals originating from tip adhesions can result in the formation of shaft adhesions, veil advance, and ultimately, growth cone movement [8].
Shaft adhesions
A single filopodium can have both non-adherent and adherent regions along the shaft. Shaft adhesions develop de novo along the filopodium and do not represent former tip adhesions [8]. Growth cone veils advance easily on non-adherent regions and cease movement as they encounter stable shaft adhesions; lateral mobility, veil advance and the merging of filopodia are also regulated by shaft adhesions [8].
Basal adhesions
Basal adhesions play a specific role in filopodia initiation and are found in ~98% of all filopodia, where they anchor the filopodial base that usually remains immobile despite considerable flexibility in the shaft [9]. These are stable adhesions that contain a focal ring structure believed to convert tension forces into filopodia formation. Basal adhesions are stable and are formed before the filopodium emerges. This has been observed in growth cones where they remain in place as the growth cone advances [9].
Increasing the force on an adhesion, either by an external source or by increasing cellular contractility, strengthens and enlarges the adhesion [10]. Similarly, in basal adhesions of filopodia in neuronal growth cones, the size and stability of nascent adhesion increases in a maturation process that is reminiscent of the focal adhesions (FAs) found in non-neuronal cells [5][6]. Though smaller than FAs, these adhesions share a number of signal components, pathways, and proteins leading to adhesion site formation and maturation [11][7][12].The structure of the growth cone adhesion site varies with the substrate [6] and adhesion directs growth cone navigation and movement (reviewed in [13][14]).
References
- Lamoureux P, Buxbaum RE, and Heidemann SR. Direct evidence that growth cones pull. Nature 1989; 340(6229):159-62. [PMID: 2739738]
- Bastmeyer M, and Stuermer CA. Behavior of fish retinal growth cones encountering chick caudal tectal membranes: a time-lapse study on growth cone collapse. J. Neurobiol. 1993; 24(1):37-50. [PMID: 8419523]
- Raich WB, Agbunag C, and Hardin J. Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr. Biol. 1999; 9(20):1139-46. [PMID: 10531027]
- Vasioukhin V, Bauer C, Yin M, and Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 2000; 100(2):209-19. [PMID: 10660044]
- Renaudin A, Lehmann M, Girault J, and McKerracher L. Organization of point contacts in neuronal growth cones. J. Neurosci. Res. 1999; 55(4):458-71. [PMID: 10723056]
- Gomez TM, Roche FK, and Letourneau PC. Chick sensory neuronal growth cones distinguish fibronectin from laminin by making substratum contacts that resemble focal contacts. J. Neurobiol. 1996; 29(1):18-34. [PMID: 8748369]
- Woo S, and Gomez TM. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J. Neurosci. 2006; 26(5):1418-28. [PMID: 16452665]
- Miki H, Sasaki T, Takai Y, and Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature 1998; 391(6662):93-6. [PMID: 9422512]
- Steketee MB, and Tosney KW. Three functionally distinct adhesions in filopodia: shaft adhesions control lamellar extension. J. Neurosci. 2002; 22(18):8071-83. [PMID: 12223561]
- Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, and Kozlov MM. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur. J. Cell Biol. 2005; 85(3-4):165-73. [PMID: 16360240]
- Yuan X, Jin M, Xu X, Song Y, Wu C, Poo M, and Duan S. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat. Cell Biol. 2003; 5(1):38-45. [PMID: 12510192]
- Luikart BW, Zhang W, Wayman GA, Kwon C, Westbrook GL, and Parada LF. Neurotrophin-dependent dendritic filopodial motility: a convergence on PI3K signaling. J. Neurosci. 2008; 28(27):7006-12. [PMID: 18596174]
- Song H, and Poo M. The cell biology of neuronal navigation. Nat. Cell Biol. 2001; 3(3):E81-8. [PMID: 11231595]
- Gallo G, and Letourneau PC. Regulation of growth cone actin filaments by guidance cues. J. Neurobiol. 2004; 58(1):92-102. [PMID: 14598373]


