How are cell-cell adhesions regulated?
Introduction to the regulation of cell-cell adhesion
The assembly and disassembly of cell-cell junctions, such as adherens junctions (AJ), are regulated by a vast array of factors functioning in a highly complex network of molecular switches and mechanical cues. Although adhesion complexes are often transient, effective regulation of their assembly and disassembly is crucial for regular cell function. This is evidenced by the fact that destabilization of AJ dynamics has been correlated to the onset of cancer cell metastasis where increased cell motility and invasion is commonly observed [1]. Indeed, one recent study attributed the oncosuppressive properties of the RhoGAP protein DLC1 (deleted in liver cancer 1) to a role in the stabilization of AJs [2].
With AJs essentially being a functional extension of the cytoskeleton, their formation and stability is heavily dependent on that of the actin filament network. Regulation of the adhesion complex is maintained by a combination of actin cytoskeleton dynamics, the influence of AJ scaffolding proteins, and the effect of post-translational modification of AJ components. Each protein involved in the regulation of the adhesion complex must first be recruited to the complex, and in most cases, must be activated in order to carry out their function. Thus, regulation of the adhesion complex is a highly complicated system involving several independent molecular pathways [3]. Efforts to dissect each regulatory mechanism are therefore ongoing and many interactions and consequences are still unknown.
How do scaffolding proteins regulate the stability of cell-cell adhesions?
Scaffolding (adaptor) proteins as regulators of adhesion stability
Scaffolding proteins are known to regulate the stability of adhesion complexes through their physical interactions with the core components. In some cases however they may also recruit additional regulatory proteins to the adhesion site. These proteins are often identified as binding partners to one or more of the key components of AJs such as E-cadherin, α-catenin and p120-catenin. In some cases scaffolding proteins, such as cortactin, may recruit regulators of actin filament dynamics like the Arp2/3 complex or N-WASP, which will regulate the function of the adhesion complex as described above. In other cases they will stabilize the core components and regulate their function.
p120-catenin regulates cell-cell adhesions stability
One adaptor protein that is crucial to the stabilization of the AJ complex is p120-catenin. This protein prevents the endocytosis of the classical cadherins in a function that was recently attributed to its binding at the juxtamembrane position of the cytoplasmic tail of cadherin [4]. Three VE-cadherin residues in particular, (DEE 646-648), which are well conserved across the classical cadherins and lie in the p120-catenin binding site, were identified as producing an endocytic signal that is blocked upon binding of p120-catenin. Mutations within this motif not only impeded p120-catenin binding but also prevented endocytosis of cadherin [4]. Similar findings have shown that binding of the small GTPase RAP1 to afadin enhances p120-catenin binding to E-cadherin, preventing endocytosis of E-cadherin and stabilizing the E-cadherein–nectin complex [5].
Additionally, an earlier study highlighted that p120-catenin may influence signaling pathways that regulate cadherin complex stability and clustering. Binding of p120-catenin to cadherin was proposed to negatively regulate Rho GTPase signaling by preventing the interaction between p120-catenin and RhoA which would otherwise result in the inhibition of RhoA activity, dissolution of actin stress fibres and the mislocalization of ezrin to the cytoplasm [6]. The latter effect is directly correlated to AJ formation and stability, with Ezrin having been shown to regulate the transport of E-cadherin to the membrane in a process that involves Rac1 activation [7]. p120-catenin’s role in regulating the Rho GTPases was again highlighted in a more recent study that indicated ROCK1 and p190A RhoGAP, which are recruited to the adhesion complex by Rho A, interact transiently with p120-catenin. These interactions may control a variety of processes including cadherin clustering and stability [8]
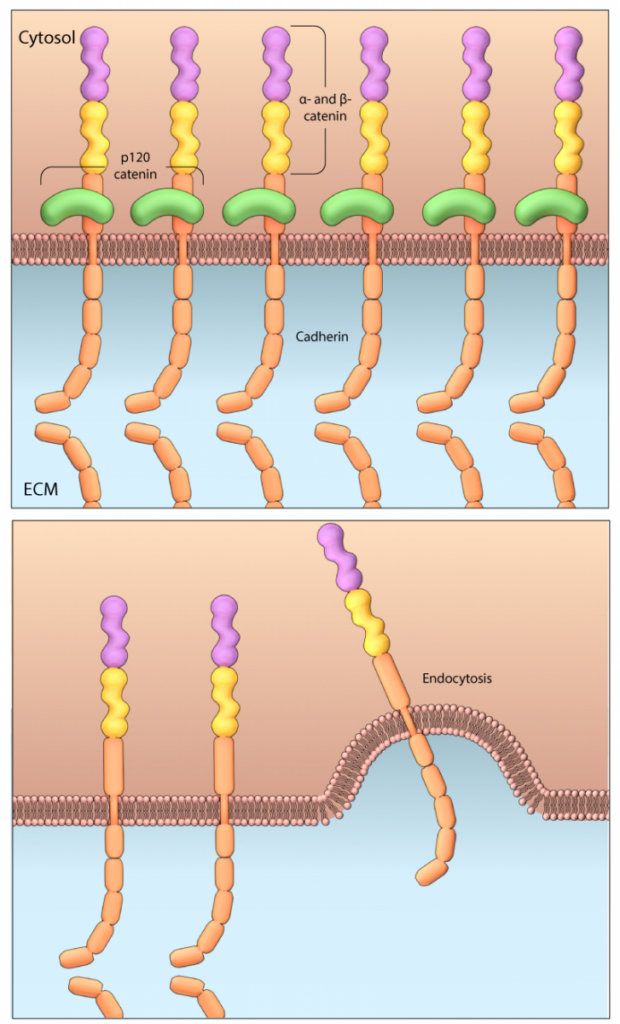
Scaffolding protein p120-catenin binds to cadherin at the juxtamembrane position of its cytoplasmic tail, which prevents endocytosis of cadherins, stabilising the cell-cell adhesions
References
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002; 2(6):442-54. [PMID: 12189386]
- Tripathi V, Popescu NC, and Zimonjic DB. DLC1 interaction with α-catenin stabilizes adherens junctions and enhances DLC1 antioncogenic activity. Mol. Cell. Biol. 2012; 32(11):2145-59. [PMID: 22473989]
- Zaidel-Bar R. Cadherin adhesome at a glance. J. Cell. Sci. 2013; 126(Pt 2):373-8. [PMID: 23547085]
- Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, and Kowalczyk AP. p120-catenin binding masks an endocytic signal conserved in classical cadherins. J. Cell Biol. 2012; 199(2):365-80. [PMID: 23071156]
- Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, and Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J. Biol. Chem. 2005; 280(25):24095-103. [PMID: 15857834]
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, and Reynolds AB. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2000; 2(9):637-44. [PMID: 10980705]
- Pujuguet P, Del Maestro L, Gautreau A, Louvard D, and Arpin M. Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol. Biol. Cell 2003; 14(5):2181-91. [PMID: 12802084]
- Smith AL, Dohn MR, Brown MV, and Reynolds AB. Association of Rho-associated protein kinase 1 with E-cadherin complexes is mediated by p120-catenin. Mol. Biol. Cell 2011; 23(1):99-110. [PMID: 22031287]


