How are actin filaments distributed in cells and tissues?
Actin filaments are widely distributed throughout cells, forming a range of cytoskeletal structures and contributing to an even broader range of processes. Some of the functions are widely observed across many cell types while in other cases actin filaments may contribute to processes that are cell type specific.
1. In polarized cells and tissues:
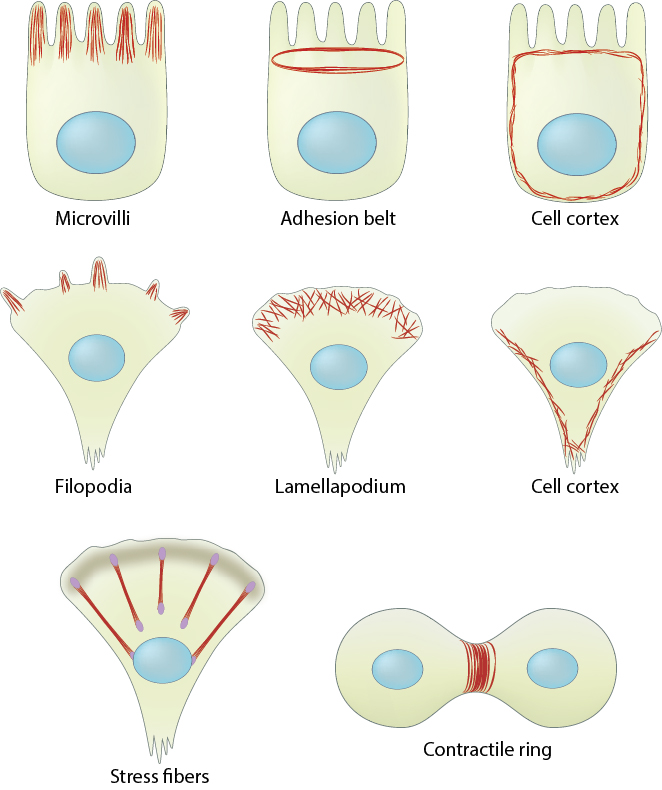
Actin filaments are widely distributed throughout cells, forming a range of cytoskeletal structures and contributing to an even broader range of processes.
Actin filaments are crucial for tissue organization and for establishing cell polarity and cohesion among epithelial cells. For example, a core of actin filaments provides microvilli structural support and enables them to increase their surface area and nutrient-absorbing capacity. These structures are found on the apical surface of epithlial cells lining the small intestine. In another example, the integrity of epithelial cell layers or sheets is maintained by a belt of actin filaments (i.e. adhesion belt). This belt links the cytoskeleton of adjacent cells. Also, certain cells use actin filament rigidity to sense vibrations, such as those found bundled on the surface of hair cells in the inner ear (called stereocilia, not shown), which tilt in response to sound. Although the actin bundles in stereocilia are stable for the lifetime of a cell (which can be decades), the individual actin filaments are continuously remodeled and replaced once every 48 hours (on average).
2. In motility-related structures:
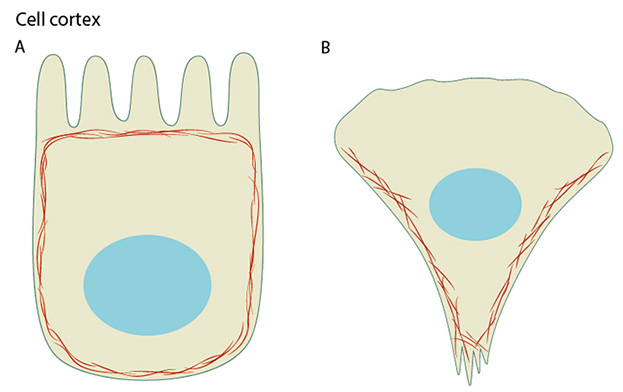
(A) Cortical actin filaments (shown in red) are concentrated just beneath the plasma membrane in most cell types. (B) Migrating fibroblasts grown in 2D tissue culture have more cortical filaments on the dorsal (upper) surface than the ventral (lower) surface and they are concentrated towards the trailing edges.
Actin filaments are the primary cytoskeletal component to drive cell motility. Here, actin filaments found in membrane protrusions such as filopodia and lamellipodia rapidly assemble and disassemble. These cellular structures are essential in cell migration and are predominately found at the leading edge of a moving cell. They also allow the cell to probe or sense its microenvironment. Actin filaments are also found at the trailing edge in the form of the cell cortex, which lies adjacent to the plasma membrane. More stable arrays of actin filaments, such as those found in stress fibers, allow a cell to brace against the underlying surface. Actin-associated myosin motor proteins use ATP hydrolysis to exert forces against the stress fibers during muscle contraction; the energy of hydrolysis can also be converted to tensile forces at the trailing cell edge to promote edge retraction in motile cells.
3. During cell division:
Actin-based motile structures are disassembled before cell division, which causes the cell to stop moving and become more rounded. More stable actin bundles remain polarized and contribute to the orientation of the microtubule network that serves as the mitotic spindle. The proper assembly, orientation, and contraction of an actin filament ring (i.e. contractile ring) serves to pinch and separate the daughter cells during cell division.
4. During reproduction:
Actin filaments are an important structural feature in the head of sperm. The rapid relaxing of coiled actin filaments during the acrosome reaction allows the sperm head to penetrate further into the egg. Microtubules influence actin assembly to help organize the polarized actin network.
How do actin filaments form higher-order assemblies that produce and respond to force?
Many different types of cells use actin filaments to generate tensional forces and to exert traction forces on their adhesions and linked ECM (or other cells); these processes cause resting tensile force (reviewed in [1][2]). Local stress or force differences that occur both internally and externally are transduced through the actin filaments and focused onto mechanosensors throughout the interlinked system rather than being limited to local deformation (reviewed in [3]); this process leads to mechanotransduction events that influence the cell shape and/or motility (reviewed in [4]).
Cells exert traction forces on the ECM and generate tension at focal adhesions through actin stress fibers, which are higher-order structures in the cytoplasm that consist of parallel contractile bundles of actin and myosin filaments. Stress fibers are linked at their ends to the ECM through focal adhesion complexes. Cell tension is generated along the actin filaments by the movement of myosin II motor proteins along the filaments (see contractile bundles). The elasticity of the actin network appears to be inherent to actin filaments and is independent of myosin II motor activity [5]. Forces produced by the contraction of stress fibers not only helps the cell body to translocate during migration [6] [7], but they also serve as a vital “inside-out? feedback system to regulate actin filament initiation [8], cell growth and motility [9][10], and formation/maturation of focal adhesion complexes [11][12]. Forces produced by stress fibers also stabilize the cell structure and contribute to establishing the cell polarity [7] and they help determine the cell fate [13][14]. There is no evidence that forces influence stress fiber contractile activity by increasing the exchange of ADP for ATP on myosin, however, force can weakly increase the release of the myosin heads from the actin filaments (reviewed in [15]).
References
- Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol. 2008; 97(2-3):163-79. [PMID: 18406455]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997; 59:575-99. [PMID: 9074778]
- Gillespie PG, and Walker RG. Molecular basis of mechanosensory transduction. Nature 2001; 413(6852):194-202. [PMID: 11557988]
- Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct 2006; 35:459-88. [PMID: 16689645]
- Chaudhuri O, Parekh SH, and Fletcher DA. Reversible stress softening of actin networks. Nature 2007; 445(7125):295-8. [PMID: 17230186]
- Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, and Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 2002; 16(10):1195-204. [PMID: 12153987]
- Svitkina TM, Verkhovsky AB, McQuade KM, and Borisy GG. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J. Cell Biol. 1997; 139(2):397-415. [PMID: 9334344]
- Gupton SL, Eisenmann K, Alberts AS, and Waterman-Storer CM. mDia2 regulates actin and focal adhesion dynamics and organization in the lamella for efficient epithelial cell migration. J. Cell. Sci. 2007; 120(Pt 19):3475-87. [PMID: 17855386]
- Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, and Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 2007; 176(5):573-80. [PMID: 17312025]
- Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, and Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat. Cell Biol. 2007; 9(3):299-309. [PMID: 17310241]
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, and Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 2001; 153(6):1175-86. [PMID: 11402062]
- Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Döbereiner H, Freund Y, Borisy G, and Sheetz MP. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell 2007; 128(3):561-75. [PMID: 17289574]
- Griffin MA, Sen S, Sweeney HL, and Discher DE. Adhesion-contractile balance in myocyte differentiation. J. Cell. Sci. 2004; 117(Pt 24):5855-63. [PMID: 15522893]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, and Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004; 6(4):483-95. [PMID: 15068789]
- Khan S, and Sheetz MP. Force effects on biochemical kinetics. Annu. Rev. Biochem. 1997; 66:785-805. [PMID: 9242924]


