Fimbrin
Fimbrin (aka plastin homologue, accumentin) is an actin binding protein that was originally identified in microvilli [1][2].
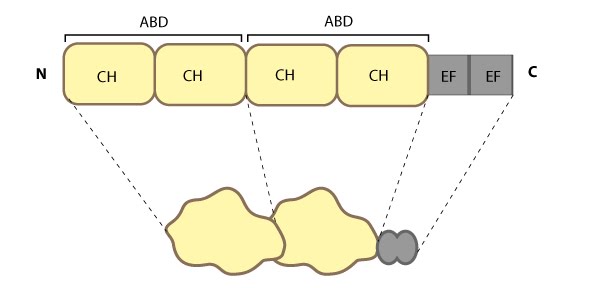
This schematic diagram illustrates the molecular organization of fimbrin as depicted in this resource, and highlights the relevant domains for binding to actin filaments.
Fimbrin represents one of the most basic structures of an actin crosslinking protein; it contains a calcium-binding domain consisting of a pair of EF hand domains, and a pair of actin binding domains (ABDs) composed of two calponin homology (CH) domains (see figure below). However no calcium sensitivity has been demonstrated for the EF domains. Fimbrin is found in a number of organisms from yeast to humans and those organisms containing multiple isoforms (e.g. humans have three) frequently exhibit isoform expression that is cell type or tissue specific (reviewed in [3][4]).
Localization and function of Fimbrin
Fimbrin primarily modulates the cytoskeleton organization of microvilli and stress fibers. Although fimbrin has been at the ends of stress fibers [5][6][7]. Fimbrin is not only dispersed throughout the lamellipodium and lamella, but it is also found in various larger actin-based structures such as stereocilia [8][9][10], cell adhesion sites, microspikes and membrane ruffles [2][11][9] and its small size allows it to crosslink actin into rigid bundles.
References
- Bretscher A, and Weber K. Fimbrin, a new microfilament-associated protein present in microvilli and other cell surface structures. J. Cell Biol. 1980; 86(1):335-40. [PMID: 6998986]
- de Arruda MV, Watson S, Lin CS, Leavitt J, and Matsudaira P. Fimbrin is a homologue of the cytoplasmic phosphoprotein plastin and has domains homologous with calmodulin and actin gelation proteins. J. Cell Biol. 1990; 111(3):1069-79. [PMID: 2391360]
- Matsudaira P. Actin crosslinking proteins at the leading edge. Semin. Cell Biol. 1994; 5(3):165-74. [PMID: 7919230]
- Bretscher A. Fimbrin is a cytoskeletal protein that crosslinks F-actin in vitro. Proc. Natl. Acad. Sci. U.S.A. 1981; 78(11):6849-53. [PMID: 6947259]
- Carley WW, Bretscher A, and Webb WW. F-actin aggregates in transformed cells contain alpha-actinin and fimbrin but apparently lack tropomyosin. Eur. J. Cell Biol. 1986; 39(2):313-20. [PMID: 3007147]
- Kamioka H, Sugawara Y, Honjo T, Yamashiro T, and Takano-Yamamoto T. Terminal differentiation of osteoblasts to osteocytes is accompanied by dramatic changes in the distribution of actin-binding proteins. J. Bone Miner. Res. 2004; 19(3):471-8. [PMID: 15040836]
- Yamashiro-Matsumura S, and Matsumura F. Intracellular localization of the 55-kD actin-bundling protein in cultured cells: spatial relationships with actin, alpha-actinin, tropomyosin, and fimbrin. J. Cell Biol. 1986; 103(2):631-40. [PMID: 3525578]
- Tilney LG, Derosier DJ, and Mulroy MJ. The organization of actin filaments in the stereocilia of cochlear hair cells. J. Cell Biol. 1980; 86(1):244-59. [PMID: 6893452]
- Messier JM, Shaw LM, Chafel M, Matsudaira P, and Mercurio AM. Fimbrin localized to an insoluble cytoskeletal fraction is constitutively phosphorylated on its headpiece domain in adherent macrophages. Cell Motil. Cytoskeleton 1993; 25(3):223-33. [PMID: 8221900]
- Marchisio PC, Cirillo D, Teti A, Zambonin-Zallone A, and Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp. Cell Res. 1987; 169(1):202-14. [PMID: 3028844]
- Yamashiro-Matsumura S, and Matsumura F. Purification and characterization of an F-actin-bundling 55-kilodalton protein from HeLa cells. J. Biol. Chem. 1985; 260(8):5087-97. [PMID: 3886649]


