Fascin
Fascin is the major actin crosslinking protein found in a wide range of filopodia [1][2][3]. This protein has been shown to work in concert with other cross linkers such as α-actinin to produce filopodia, although fascin itself is sufficient to form filopodia-like bundles in a reconstitution system [4]. Moreover, fascin and α-actinin are observed to co-localize at the base of filopodia, where together they can produce a mechanical response of greater magnitude than when acting alone [5]. Cells harboring defective fascin create less filopodia and their mechanical properties are drastically compromised [6]. In lamellipodia-like structures, fascin is suggested to organize the actin cytoskeleton at the leading edge to promote cell motility [7].
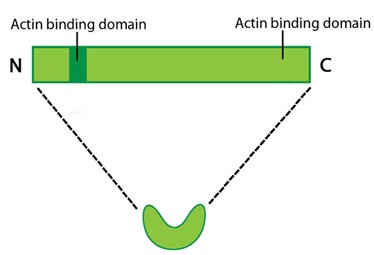
A schematic of fascin, as the molecule is depicted throughout MBInfo
Protein structure A 55kDa globular protein that is a component of the crosslinking actin filaments functional module. Fascin organizes F-actin into tightly packed parallel bundles approximately 8nm apart. It also promotes side-branching of actin filaments and can crosslink actin filaments without forming bundles [8]. Metazoans contain three fascin forms (e.g. fascin-1, -2, and -3) encoded by three separate genes while invertebrates contain a single form of fascin (reviewed in [9]). Fascin contains two actin-binding domains (ABD), one located at each end of the protein (see figure below) [10][11][12]. Fascin binds to actin filaments with a stoichiometry in a range of one molecule of fascin to 2-3 molecules of actin (in brain tissue) [1] or 1:4 (in vitro) [2].
Localization and function of Fascin
A steady pool of F-actin monomers or loosely linked F-actin promotes efficient polymerization and bundling of actin-filaments by fascin (reviewed in [9]). Fascin phosphorylation at the amino-terminal actin binding site by protein kinase Cα (PKCα) inhibits its ability to bind actin (at both sites) and to form bundles [10][11]. Although fascin undergoes frequent cycles of association and dissociation rather than binding to actin filaments stably, this process is independent of the phosphorylation state [6]. The rate at which fascin dissociates from actin is slow and appears to contribute to the reduced movement of actin filaments and bundles i.e. increased stiffness of the actin network [8].
Fascin is found in the most distal regions of filopodia and lamellipodia and the cellular distribution of fascin within actin bundles (e.g. microspikes and stress fibers [13]) appears to vary depending upon the extracellular substrate [14][15][16]; however, specific details about how this distribution and assembly on the actin filaments is regulated remains largely unknown [6].
References
- Sasaki Y, Hayashi K, Shirao T, Ishikawa R, and Kohama K. Inhibition by drebrin of the actin-bundling activity of brain fascin, a protein localized in filopodia of growth cones. J. Neurochem. 1996; 66(3):980-8. [PMID: 8769857]
- Yamashiro-Matsumura S, and Matsumura F. Purification and characterization of an F-actin-bundling 55-kilodalton protein from HeLa cells. J. Biol. Chem. 1985; 260(8):5087-97. [PMID: 3886649]
- Ross R, Jonuleit H, Bros M, Ross XL, Yamashiro S, Matsumura F, Enk AH, Knop J, and Reske-Kunz AB. Expression of the actin-bundling protein fascin in cultured human dendritic cells correlates with dendritic morphology and cell differentiation. J. Invest. Dermatol. 2000; 115(4):658-63. [PMID: 10998139]
- Vignjevic D, Yarar D, Welch MD, Peloquin J, Svitkina T, and Borisy GG. Formation of filopodia-like bundles in vitro from a dendritic network. J. Cell Biol. 2003; 160(6):951-62. [PMID: 12642617]
- Tseng Y, Kole TP, Lee JSH, Fedorov E, Almo SC, Schafer BW, and Wirtz D. How actin crosslinking and bundling proteins cooperate to generate an enhanced cell mechanical response. Biochem. Biophys. Res. Commun. 2005; 334(1):183-92. [PMID: 15992772]
- Vignjevic D, Kojima S, Aratyn Y, Danciu O, Svitkina T, and Borisy GG. Role of fascin in filopodial protrusion. J. Cell Biol. 2006; 174(6):863-75. [PMID: 16966425]
- Yamashiro S, Yamakita Y, Ono S, and Matsumura F. Fascin, an actin-bundling protein, induces membrane protrusions and increases cell motility of epithelial cells. Mol. Biol. Cell 1998; 9(5):993-1006. [PMID: 9571235]
- Tseng Y, Fedorov E, McCaffery JM, Almo SC, and Wirtz D. Micromechanics and ultrastructure of actin filament networks crosslinked by human fascin: a comparison with alpha-actinin. J. Mol. Biol. 2001; 310(2):351-66. [PMID: 11428894]
- Kureishy N, Sapountzi V, Prag S, Anilkumar N, and Adams JC. Fascins, and their roles in cell structure and function. Bioessays 2002; 24(4):350-61. [PMID: 11948621]
- Yamakita Y, Ono S, Matsumura F, and Yamashiro S. Phosphorylation of human fascin inhibits its actin binding and bundling activities. J. Biol. Chem. 1996; 271(21):12632-8. [PMID: 8647875]
- Ono S, Yamakita Y, Yamashiro S, Matsudaira PT, Gnarra JR, Obinata T, and Matsumura F. Identification of an actin binding region and a protein kinase C phosphorylation site on human fascin. J. Biol. Chem. 1997; 272(4):2527-33. [PMID: 8999969]
- Edwards RA, Herrera-Sosa H, Otto J, and Bryan J. Cloning and expression of a murine fascin homolog from mouse brain. J. Biol. Chem. 1995; 270(18):10764-70. [PMID: 7738015]
- Yamashiro-Matsumura S, and Matsumura F. Intracellular localization of the 55-kD actin-bundling protein in cultured cells: spatial relationships with actin, alpha-actinin, tropomyosin, and fimbrin. J. Cell Biol. 1986; 103(2):631-40. [PMID: 3525578]
- Adams JC. Formation of stable microspikes containing actin and the 55 kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the anti-adhesive activities of thrombospondin-1. J. Cell. Sci. 1995; 108 ( Pt 5):1977-90. [PMID: 7657718]
- Adams JC. Characterization of cell-matrix adhesion requirements for the formation of fascin microspikes. Mol. Biol. Cell 1997; 8(11):2345-63. [PMID: 9362073]
- Fischer D, Tucker RP, Chiquet-Ehrismann R, and Adams JC. Cell-adhesive responses to tenascin-C splice variants involve formation of fascin microspikes. Mol. Biol. Cell 1997; 8(10):2055-75. [PMID: 9348542]


