Regulating Neuron Growth
Lakshmi Ramachandran | September 2015
MBI scientists discover the molecular mechanism behind neuronal signaling via the neurotransmitter acetylcholine (ACh), which is critical for brain function and development. This work is published in Developmental Cell today [Sun et al, BNIP-H recruits the cholinergic machinery to neurite terminals to promote acetylcholine signalling and neuritogenesis. Developmental Cell, dx.doi.org/10.1016/j.devcel.2015.08.006].
BNIP-H regulates neurotransmitter Acetylcholine
Our body is wired by a network of nerves that transmit information in the form of electric signals to and from the brain, eventually dictating all of our actions. Key to this transmission of nerve impulses are a class of chemicals commonly referred to as neurotransmitters. Often found at synapses, which are the junctions between individual nerve cells or neurons, neurotransmitters relay information from one neuron to another. There are different types of neurotransmitters performing a variety of roles, ranging from controlling muscle movement, memory and even our mood.
The first neurotransmitter discovered, ACh, is required for critical functions like muscle stimulation, learning and memory. Therefore a drop in ACh levels is, not surprisingly, associated with movement disorders like paralysis, as well as neurological diseases such as dementia and Alzheimer’s disease. From extensive research on ACh, we know that at the molecular level it promotes the development of a nerve cell through a process known as neurite outgrowth. During this process, multiple dendrites and a single long axon begin to project out from the nerve cell body, giving the neuron its typical shape.
Spatial regulation of Acetylcholine
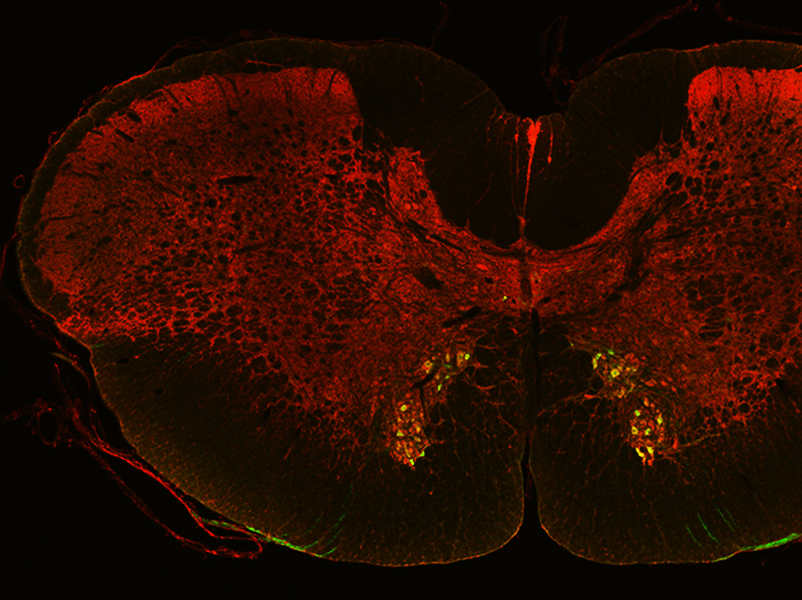
However, the spatial regulation of ACh synthesis remained unclear until Jichao Sun, a postdoctoral researcher from the lab of Associate Professor Boon Chuan Low at the Mechanobiology Institute (MBI), National University of Singapore, discovered that a protein called BNIP-H regulates the precise localization of the metabolic enzymes ATP citrate lyase (ACL) and choline acetyltransferase (ChAT), which are responsible for ACh synthesis.
MBI researchers shed light on drop in ACh levels and signaling that are a hallmark of Alzheimer’s disease and dementias, which could lead to new treatments.
They found that BNIP-H associated with a motor protein known as kinesin. Kinesin is well known for transporting cellular cargo over large distances, for instance, from one end of a nerve cell to the other, by ‘walking’ along ‘tracks’ formed by long filamentous structures called microtubules. While BNIP-H moves along the neurites via kinesin, it also acts as a scaffold, forming a complex with ACL. Once this complex arrives at neurite terminals, ChAT is recruited for ACh synthesis and release, triggering a signaling pathway that promotes and reinforces neurite outgrowth.
Extending their studies into living organisms, the scientists found that mutation of the BNIP-H gene in zebrafish causes disrupted ACh signaling. This led to impaired neurite outgrowth, abnormal development of motor neurons, and consequently these fish exhibited severe movement defects. Interestingly, mutation of BNIP-H in humans can cause a rare genetic disorder called Cayman ataxia. This disease is characterized by inability to coordinate and control muscular movements, similar to the symptoms seen in the BNIP-H mutant zebrafish.
This study reveals how BNIP-H spatially regulates ACh to eventually promote neurite outgrowth. The discovery that Cayman ataxia could arise from dysregulation of this molecular mechanism provides new avenues for investigation and therapy. Promisingly, this mechanism may also shed light on the significant drop in ACh levels and signaling that are a hallmark of Alzheimer’s disease and dementias, which could lead to novel drug targets to treat these debilitating disorders.
Sun, JC, Pan, CQ, Chew, TW, Liang, FY, Burmeister, M and Low, BC (2015) BNIP-H recruits the cholinergic machinery to neurite terminals to promote acetylcholine signalling and neuritogenesis. Developmental Cell 34(5): 555–568.
In the news
This research has been featured in the following outlets:
- NUS News: Coordinating traffic down the neuronal highway
- Science Daily: Coordinating traffic down the neuronal highway
- University of Michigan: Michigan Medicine: Collaborative global study aims to enhance understanding of rare motor disorder
- The Straits Times: Beautiful Science